More Information
Submitted: May 09, 2022 | Approved: May 24, 2022 | Published: May 25, 2022
How to cite this article: Marangoz E, Yüksel D, Yaylalı O, Eskiçorapçı SY, Şen N, et al. The value of bone scans to predict survival time in patients with diagnosed prostate cancer: single-center retrospective study. J Radiol Oncol. 2022; 6: 004-011.
DOI: 10.29328/journal.jro.1001040
Copyright License: © 2022 Marangoz E, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Prostate cancer; Bone scintigraphy; Survival; Bone metastases
The value of bone scans to predict survival time in patients with diagnosed prostate cancer: single-center retrospective study
Elif Marangoz1, Doğangün Yüksel1* , Olga Yaylalı1, Saadettin Yılmaz Eskiçorapçı2, Nilay Şen3, Hülya Aybek4 and Fatma Suna Kıraç1
, Olga Yaylalı1, Saadettin Yılmaz Eskiçorapçı2, Nilay Şen3, Hülya Aybek4 and Fatma Suna Kıraç1
1Department of Nuclear Medicine, Faculty of Medicine, Pamukkale University, Denizli, Turkey
2Department of Urology, Faculty of Medicine, Pamukkale University, Denizli, Turkey
3Department of Pathology, Faculty of Medicine, Pamukkale University, Denizli, Turkey
4Department of Biochemistry, Faculty of Medicine, Pamukkale University, Denizli, Turkey
*Address for Correspondence: Dr. Doğangün Yüksel, Professor, Department of Nuclear Medicine, Faculty of Medicine, Pamukkale University, PAU Tıp Fakültesi Nükleer Tıp A.D. PAU SARUM Kınıklı, 200160, Denizli, Turkey, Email: [email protected]
Objective: In this study, we investigated the significance of the bone scan results as a prognostic factor to predict survival by comparing age, serum PSA level, and Gleason score.
Methods: Medical records of 313 patients were retrospectively examined. 265 patients of 313 were included in the study.
Results: 202 (76%) patients of 265 were still alive and 63 (24%) patients of 265 were dead because of prostate cancer. Patients’ mean estimated survival times for those with, without, and suspected bone metastases were 47.4 ± 5.4 months, 159.1 ± 8.6 months, and 71.1 ± 14.4 months, respectively (p = 0.0001). While the mean estimated survival time of < 70 years patients old was 137.1 ± 9.4 months, the mean estimated survival time of ≥ 70 years old patients was 78.2 ± 5.0 (p = 0.031). 243 patients with known PSA values, of those whose PSA levels were < 10 ng/ml, between 10-20 ng/ml, between > 20-50 ng/ml, and > 50 ng/ml, the estimated mean survival time was 106.9 ± 4.2 months, 118.1 ± 14.8 months, 87.6 ± 7.4 months and 51.7 ± 6.2 month, respectively and a significant difference was determined (p = 0.0001). For patients whose Gleason scores were < 7, 7, and >7, the mean estimated survival time was 167.5 ± 10.8 months), 86.8 ± 5.5 months, and 61.0 ± 5.4 months, respectively, and a significant difference was determined (p = 0.0001).
Conclusion: We identified that the estimated mean survival time of the patients who had bone metastases, had a high level of PSA, had a high level of Gleason score, and were older than 70 years old was shorter than other groups. We concluded the most important prognostic factor affecting survival time independently was the finding of metastasis detected in bone scintigraphy.
Prostate cancer is the most common cancer in men and is the second leading cause of cancer-related death after lung cancer. It is estimated that there are 174,650 new diagnoses and 31,620 prostate cancer deaths in the USA in 2019 [1].
Prostate cancer and lung cancer are the most common cancers that metastasize to the bone in men. Bone scintigraphy, which is a sensitive method, is generally used to detect bone metastases [2]. In determining the indication for bone scintigraphy, an approach based on PSA value and Gleason score is generally preferred [3-11].
The main prognostic factors used in the prediction of disease-free period and survival time are PSA value, pathological stage, and clinical stage [3,4,12]. One of the prognostic factors is bone scintigraphy findings. In several studies, it has been suggested that the number or spread of metastatic bone lesions detected in bone scintigraphy in patients with metastatic prostate cancer reflects the prognosis [13,14]. There are very few studies comparing patients with bone metastasis on bone scintigraphy and patients without bone scintigraphy [15].
In this retrospective study, we investigated the value of bone scintigraphy results in predicting survival time compared to age, serum PSA level, and Gleason score as a prognostic factor, including patients without bone metastasis.
The study used clinical information from the patients and was approved by the Medical Ethics Committee at University (approval number: 17.01.2012/2). Patients or their relatives were reached for survival analysis and their consent was obtained.
The files of 313 patients with prostate adenocarcinoma who were referred to the department of nuclear medicine for whole-body bone scintigraphy were retrospectively analyzed.
Age, Gleason score, and total PSA values before scintigraphy of the patients were recorded. The patients were called by phone. Whether they were alive or not, if they died, the cause and time of death were learned. Among the patients who died, patients with additional malignancies besides prostate cancer and those who died due to another reason (cerebrovascular accident, ischemic heart disease, accidents, etc.) were excluded from the study.
The survival times of the patients included in the study were calculated in months. To investigate the effect of age on survival time, patients were classified as under 70 years of age, 70 years, and above.
Patients who are informed orally and in writing before the whole-body bone scintigraphy sign a written consent form that they accept the procedure. In our department, 740 MBq Tc-99m MDP is administered intravenously to patients for whole-body bone scintigraphy. Patients are scanned 2-4 hours after injection. The imaging procedure is performed as whole-body scanning using a single-headed CamStar AC/T gamma camera (GE-Milwaukee, Wisc., USA) and a low-energy general-purpose collimator in the supine position. The scanning speed is 10 min/m, and the imaging matrix is 1024 x 512. Images were evaluated visually.
The patients were divided into 3 groups according to the results of bone scintigraphy cases with metastasis to the skeletal system, no metastasis, and with suspicious metastasis. In the skeletal system, randomly distributed, multiple, increased focal activity involvement in different sizes, different intensities, and diffuse, intense activity involvement (super scan) were interpreted as skeletal system metastasis. The scintigraphic findings of patients with single focal activity involvement without a history of trauma were considered suspicious. The scintigraphic findings of these patients who were found to have metastasis corresponding to the suspicious lesion on scintigraphy with other imaging methods such as plain X-ray, CT, and MRI were interpreted as metastasis. The scintigraphic findings of the patients who have not applied any other imaging method for correlative purposes were interpreted as suspicious metastasis because the patient had a definite diagnosis of prostate cancer.
Total PSA levels are measured on the same day using the chemiluminescence method on the autoanalyzer (Roche Cobas 6000, Roche-Hitachi Diagnostics, Japan) from the serum samples obtained. According to this method, the PSA reference range was 0-4 ng/ml. The patients were divided into 4 groups according to their serum PSA levels as < 10 ng/ml, 10-20 ng/ml, > 20-50 ng/ml, > 50 ng/ml [4,6,9]. Serum PSA levels of 22 patients were not known before bone scintigraphy.
Pathological preparations (TRIB, TUR-P, prostatectomy) of the patients were evaluated based on the Gleason system. According to this system, the structural pattern of the tumor is graded from the best differentiation being one and the worst differentiation being five. The Gleason score is obtained by summing the differentiation degrees of the most common and second common patterns. The patients were divided into 3 groups according to their Gleason scores as < 7, 7, and > 7 [16,17].
Statistical Package for the Social Sciences (SPSS) 17.0 program was used for statistical analysis. Frequency analysis of variables was done. Continuous variables were shown as mean ± standard deviation (SD). The number of patients in variables and their subgroups was shown as number (n) and percentage (%).
Nonparametric methods were used because the distribution of data did not conform to normal distribution. Kruskal Wallis Analysis of Variance was used to determine whether there was a statistically significant difference between variable subgroups. If there was a statistically significant difference between more than two groups, paired analyzes were performed using the Bonferroni-corrected Mann Whitney U Test to find out which group or groups the difference originated from.
Patients’ lifetimes were calculated in months, with death as the endpoint. Kaplan-Meier method was used for survival analysis. Survival curves were compared using the log-rank test. The predicted mean survival time found by the Kaplan-Meier method was defined as “mean ± standard error”. In addition, the predicted median survival time was calculated due to the heterogeneous distribution of the patients in subgroups formed according to bone scintigraphy findings.
Multivariate Cox regression analysis was used to find independent prognostic factors affecting survival. Age, serum PSA level, Gleason score, and Tc-99m MDP whole-body bone scintigraphy findings were included in the analysis. The prognostic effect level was indicated by the Wald value calculated for factors with a p - value less than 0.05.
The files of 313 patients with prostate cancer who underwent bone scintigraphy examination were analyzed from the archive of the Department of Nuclear Medicine. A total of 48 patients were excluded from the study. The remaining 265 patients constituted our study group.
Of the 265 patients included in the study, 202 (76%) were still alive, and 63 (24%) patients were found to have died due to prostate cancer. The distribution of patients in variable subgroups and average life span is shown in Table 1.
| Table 1: Patient distribution and average life expectancy in variable subgroups. | |||||
| Variable | Subgroups | n | % | Mean Survive (months) | p - value* |
| Bone scan | Metastasis yes | 70 | 26 | 31.8 ± 24.0 | 0.0001 |
| Suspected metastasis | 23 | 9 | 38.1 ± 26.3 | ||
| Metastasis no | 172 | 65 | 45.8 ± 28.0 | ||
| Age (years) | < 70 | 134 | 51 | 46.5 ± 30.5 | 0.02 |
| ≥ 70 | 131 | 49 | 36.1 ± 22.9 | ||
| PSA (ng/ml) | < 10 | 93 | 38 | 40.9 ± 22.2 | 0.008 |
| 10-20 | 48 | 20 | 41.8 ± 24.7 | ||
| > 20-50 | 40 | 16 | 42.2 ± 26.6 | ||
| > 50 | 62 | 26 | 31.3 ± 24.1 | ||
| Unknown | 22 | ||||
| Gleason Score | < 7 | 96 | 40 | 46.4 ± 28.1 | 0,004 |
| 7 | 65 | 27 | 38.0 ± 23.2 | ||
| > 7 | 79 | 33 | 35.1 ± 25.3 | ||
| Unknown | 25 | ||||
| *Kruskal Wallis analysis of variance. | |||||
In bone scintigraphy, metastasis to the skeletal system was not detected in 172 (65%) patients, while metastasis was found in 70 (26%) patients. The remaining 23 (9%) patients had suspicious findings for metastasis.
According to the presence of bone metastases in bone scintigraphy, the average life expectancy after diagnosis was determined in 172 patients without metastasis at 45.8 ± 28.0 months (range 5.2 - 182.9 months), in 70 patients with metastasis at 31.8 ± 24.0 months (range 0.3 - 106.8 months) and in 23 patients with suspected metastasis 38.1 ± 26.3 months (range 2.5 - 119.6 months) (p = 0.0001). A very significant difference was found between patients with and without bone metastasis (p = 0.0001). There was no statistically significant difference between the group without bone metastasis and the group with suspicious metastasis (p = 0.151) and between the group with bone metastasis and the group with suspicious metastasis (p = 0.297).
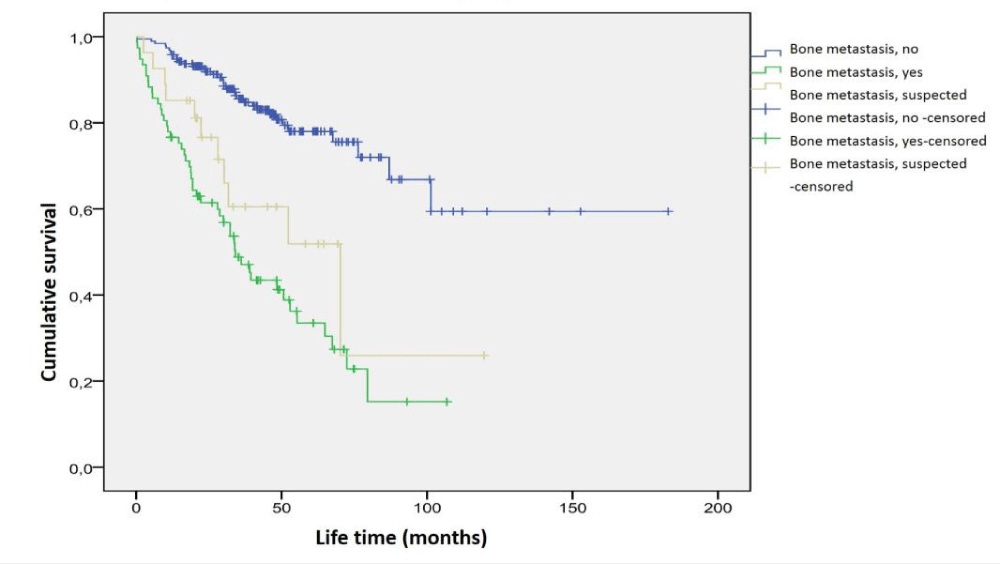
In Kaplan-Meier analysis, 15 (9%) of 172 patients without metastasis in bone scintigraphy, 41 of 70 patients (59%) with bone metastasis, and 7 (30%) of 23 patients who were considered suspicious for bone metastasis, it was seen that they died due to cancer. Mean survival time calculated according to the presence of bone metastases is as follows; In 172 patients with no metastasis in bone scintigraphy, 159.1 ± 8.6 months (95% CI: 142.2 - 176.0 months), in 70 patients with metastasis in bone scintigraphy 47.4 ± 5.4 months (95% CI: 36.7 - 58.0 months), and 23 patients with suspicious metastasis in bone scintigraphy 71.1 ± 14.4 months (95% CI: 42.8 - 99.4 months). There was a statistically significant difference between these three groups in terms of predicted mean survival times (p = 0.0001). The average survival time predicted by the presence of bone metastasis in the patients is shown in Figure 1.
Figure 1: Survival time analysis according to the presence of bone metastasis by scintigraphy.
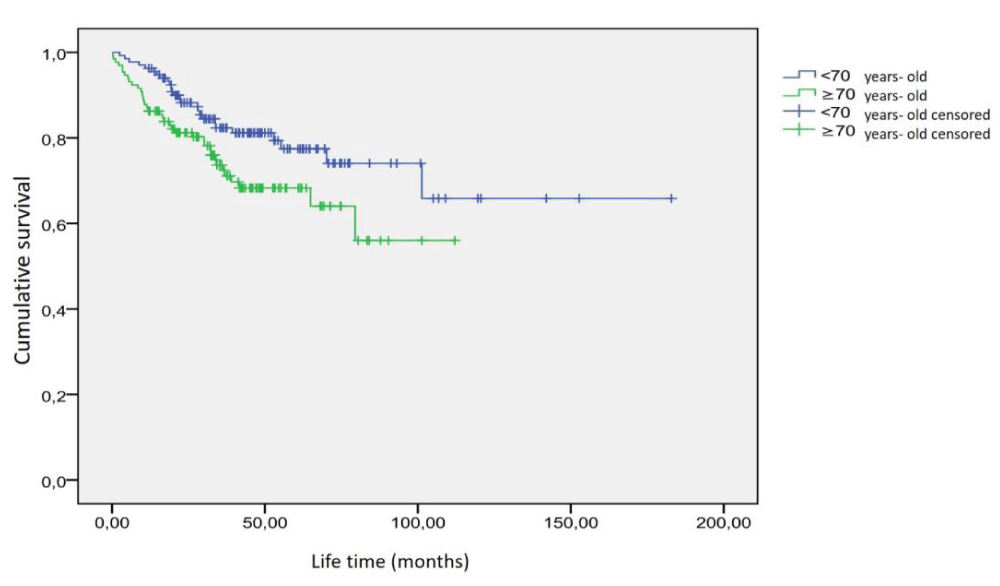
The mean age of 265 patients included in the study was 68.6 ± 8.3 years (range 46 - 90 years). Of the patients, 134 (51%) were under the age of 70, and 131 (49%) were 70 years and over. The average life expectancy of 134 patients under the age of 70 was 46.5 ± 30.5 months (range 2.5 - 182.9 months), and the average life expectancy of 131 patients aged 70 years and over was 36.1 ± 22.9 months (range 0.3 - 112.1 months). There was a significant difference (p = 0.02). 26 (20%) of 134 patients under the age of 70 died due to prostate cancer. The predicted mean survival time was 137.1 ± 9.4 months (95% CI: 118.8 - 155.5 months). It was observed that 37 (28%) of 131 patients aged 70 and over died due to prostate cancer. The predicted mean survival time in these patients was 78.2 ± 5.0 months (95% CI: 68.5 - 87.0 months). A statistically significant difference was found between these two groups in the survival analysis (p = 0.031). The graphic of the survival time analysis according to the age groups of the patients is shown in Figure 2.
Figure 2: Survival time analysis as age groups.
PSA values of 22 patients were not known. The mean PSA value of 243 patients with known PSA values before scintigraphy was 94.2 ± 413.1 ng/ml (range 0.003 - 5459 ng/ml). Among these patients, the mean PSA value of patients with metastasis on bone scintigraphy was 285.7 ± 783.2 ng/ml (Range 0.02 - 5492 ng/ml), and the mean PSA value of patients without metastasis on bone scintigraphy was 26.8 ± 42.8 ng/ml (range 0.003 - 324.24 ng/ml) and the mean PSA value of the patients with suspicious metastasis on bone scintigraphy was calculated as 30.2 ± 35.7 ng/ml (range 0.001-108.88 ng/ml).
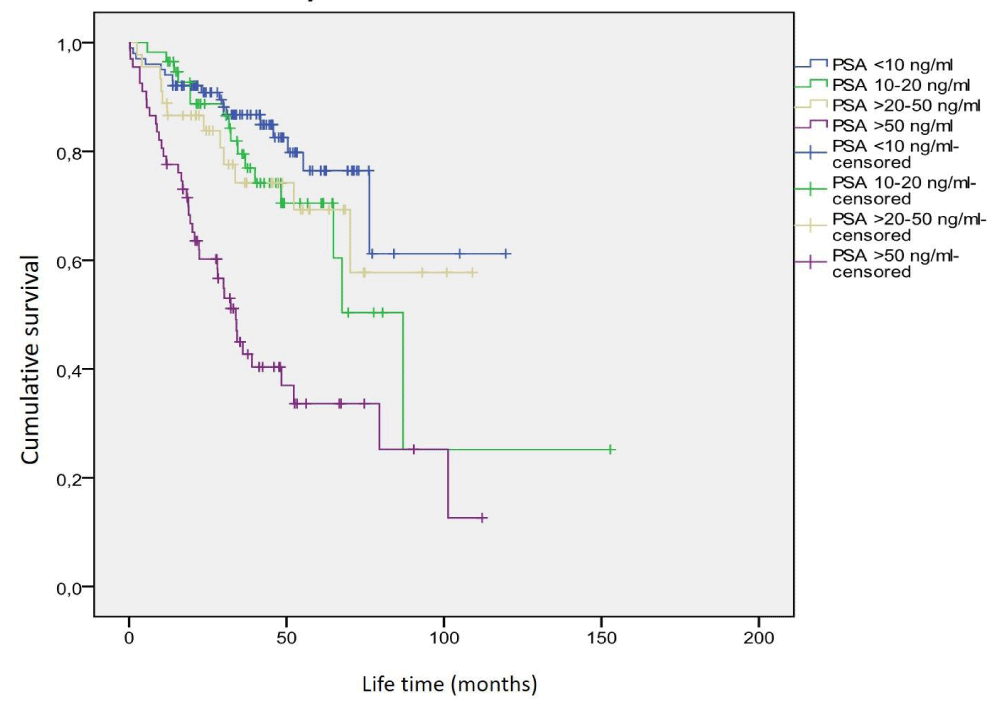
Mean survival time was 40.9 ± 22.2 months (range 0.3 - 119.6 months) in 93 patients (38%) with PSA level < 10 ng/ml, 41.8 ± 24.7 months in 48 patients (20%) with PSA level 10-20 ng/ml (range 11.8 - 152.8 months), 42.2 ± 26.6 months (range 2.5 - 109 months) in 40 patients (16%) with PSA level > 20 - 50 ng/ml, and 31.3 ± 24.1 months in 62 patients (26%) with PSA level > 50 ng/ml (range 0.4 - 112.1 months) and a statistically significant difference was found between these groups (p = 0.008). In paired comparisons, there were statistically significant differences between the group with PSA level < 10 ng/ml and the group with PSA level > 50 ng/ml (p = 0.002), between the group with a PSA level of 10-20 ng / ml and the group with a PSA level of > 50 ng/ml (p = 0.004), and between the group with PSA level > 20 - 50 and the group with PSA level > 50 ng/ml (p = 0.024).
In Kaplan-Meier analysis, it was seen that 9 (10%) of 93 patients with PSA levels < 10 ng/ml died due to prostate cancer. The predicted mean survival time was calculated as 106.9 ± 4.2 months (95% CI: 98.7 - 115.0 months). It was observed that 7 (15%) of 48 patients with a PSA level of 10-20 ng/ml died due to prostate cancer. The predicted mean survival time was calculated as 118.1 ± 14.8 months (95% CI: 89.1-147.1 months). It was observed that 7 (18%) of 40 patients with PSA levels> 20-50 ng/ml died due to prostate cancer. The predicted mean survival time was calculated as 87.6 ± 7.4 months (95% CI: 73.1-102.2 months). It was observed that 35 (56%) of 62 patients with PSA levels > 50 ng/ml died due to prostate cancer. The predicted mean survival time was calculated as 51.7 ± 6.2 months (95% CI: 39.6 - 63.8 months). Statistically significant differences were found between the groups in terms of survival (p = 0.0001). The graphic of the survival time analysis according to the PSA levels of the patients is shown in Figure 3.
Figure 3: Survival analysis according to PSA level.
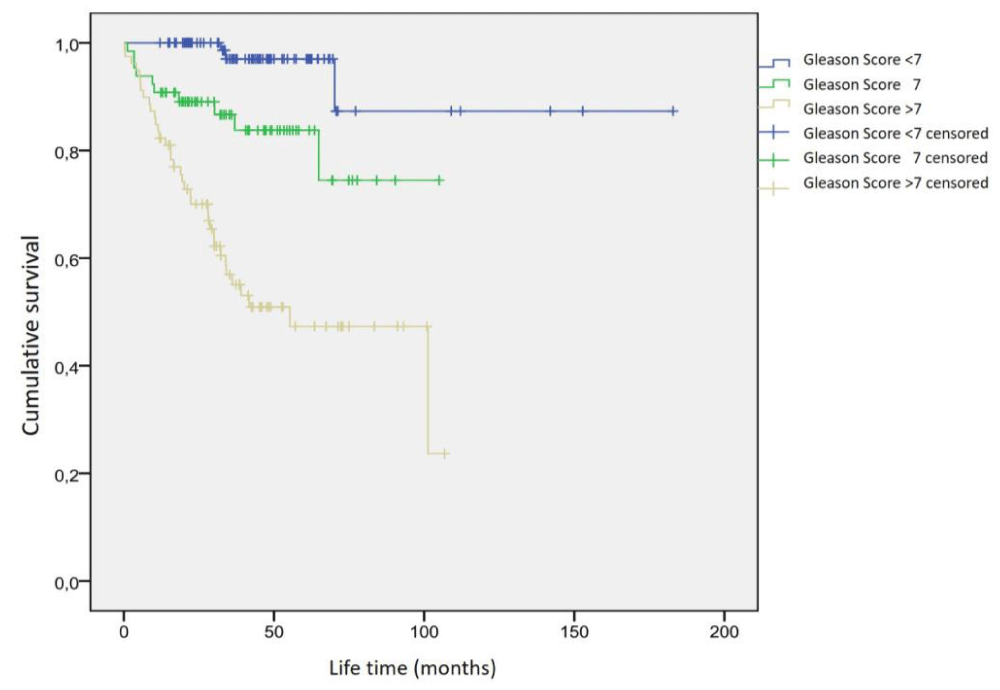
Of the 265 patients included in the study, there were 96 (40%) with Gleason scores < 7, 65 (27%) with 7, and 79 (33%) with > 7. The Gleason score of the remaining 25 patients was unknown. The lowest Gleason score of the patients was 2 and the highest 10. Considering the Gleason score, the mean life expectancy of 96 patients with a Gleason score < 7 was 46.4 ± 28.1 months (Range 12-182.9 months), 65 patients with a Gleason score of 7 were 38.0 ± 23.2 months (Range 1.2 - 105.0 months). For the Gleason score > 7, it was calculated as 35.1 ± 25.3 months (Range 0.3 - 106.8 months) for 79 patients. A significant difference was observed between the mean life span of these three groups (p = 0.004). In the dual analysis of subgroups in terms of Gleason score, the most significant difference in terms of survival was found between the groups with Gleason scores < 7 and > 7 (p = 0.001). There was no statistically significant difference between the groups with Gleason scores < 7 and 7 (p = 0.054) and between groups with Gleason scores > 7 and 7 (p = 0.278).
In Kaplan-Meier analysis, 3 (3%) of 96 patients with Gleason scores < 7 died due to prostate cancer. The predicted mean survival time was 167.5 ± 10.8 months (95% CI: 146.4-188.6 months). It was observed that 10 (15%) of 65 patients with a Gleason score of 7 died due to prostate cancer. The predicted mean survival time was 86.8 ± 5.5 months (95% CI: 76.0 - 97.6 months). It was observed that 36 (46%) of 79 patients with Gleason score> 7 died due to prostate cancer. The predicted mean survival time was 61.0 ± 5.4 months (95% CI: 50.5 - 71.5 months). A statistically significant difference was found in the survival analysis between these three groups (p = 0.0001). The graphic of the survival time analysis according to the Gleason score groups is shown in Figure 4.
Figure 4: Survival analysis of patients in the Gleason score subgroups.
In Cox regression analysis (Table 2), it was seen that the most important factor independently affecting survival was bone scintigraphy findings (Wald = 19.70; p = 0.0001). The Gleason score (Wald = 13.25; p = 0.001) and PSA level (Wald = 9.05; p = 0.029), although less potent, were observed as independent prognostic factors. Age (Wald = 0.332; p = 0.564) was found to have no significant effect on prognosis.
| Table 2: Evaluation of prognostic factors by Cox regression analysis. | ||||||
| 95% CI for HR§ | ||||||
| Variable | SE* | Wald | p - value | HRŧ | Lower limit | Upper limit |
| Age | 0.317 | 0.332 | 0.564 | 0.833 | 0.447 | 1.551 |
| Gleason score | 13.247 | 0.001 | ||||
| = 7 | 0.754 | 11.534 | 0.001 | 0.077 | 0.018 | 0.339 |
| > 7 | 0.392 | 3.679 | 0.055 | 0.471 | 0.218 | 1.017 |
| PSA | 9.053 | 0.029 | ||||
| 10-20 ng/ml | 0.422 | 7.231 | 0.007 | 0.322 | 0.141 | 0.735 |
| > 20 - 50 ng/ml | 0.475 | 2.307 | 0.129 | 0.486 | 0.192 | 1.233 |
| > 50 ng/ml | 0.507 | 3.056 | 0.08 | 0.412 | 0.153 | 1.113 |
| Bone scan | 19.7 | 0.0001 | ||||
| Metastasis suspected | 0.363 | 18.924 | 0.0001 | 0.206 | 0.101 | 0.42 |
| Metastasis positive | 0.48 | 0.088 | 0.767 | 0.867 | 0.339 | 2.22 |
| *Standard error; ŧHazard ratio; §Confidence interval | ||||||
Distant metastasis was detected in at least 40% of prostate cancer patients in the clinic at the time of diagnosis. About 80% of patients who died due to prostate cancer have skeletal system metastasis [18]. Bone metastases are an important problem in many patients with advanced prostate cancer. The probability of annual bone metastasis development in patients with advanced-stage prostate cancer is 8%, and this rate increases to 40% within five years [19]. Radionuclide bone scintigraphy is the most useful diagnostic method for the detection of bone metastases because it is a sensitive, cheap, easily accessible, and applicable method, and also can perform whole-body scanning without additional radiation exposure with single imaging [20].
In our study, we detected bone metastasis in 26% of the patients (70 of 265 patients) with whole-body bone scintigraphy. The rate of metastasis detection in bone scintigraphy in patients diagnosed with prostate cancer is given in a wide range from 2.5% to 61% in the literature [5-11,21-28]. The closest values to the bone metastasis rate in our study were found by Kosuda, et al. [6] (22%; 287 of 1294 patients) from Japan and Lai, et al. [23] (29%; 34 of 116 patients) from China. The metastasis rates found in bone scintigraphy were reported by Salonia, et al. [27] reported 2.5% for the Italian population (31 of 1242 patients), while Ritenour, et al. [28] reported lower levels of 4% (32 out of 800 patients) for the American population. Bone metastasis rates in our patient group are closer to those reported in Asian countries. Considering that the patients in our study group belonged to the Asian-Caucasian race, this finding is an expected finding.
In the literature, we found the highest rate of bone metastases (61%) reported in a study by Tekdoğan, et al. [11]. Compared to their study, the important difference is the rate of our patients with PSA levels above 50 ng/ml. The proportion of patients (50%) in their study was almost twice that of ours (23%). Another difference is the number of patients. The number of patients (99 patients) in the studies of Tekdoğan, et al. [11] is less than the number of patients in our study (265 patients). That mentioned study of higher bone metastasis with a lower number of patients compared to us suggests that patients with higher PSA levels were selected and sent for bone scintigraphy, considering that the possibility of bone metastasis will increase as the PSA level increases.
The difference between our study from many studies in the literature is that we also included cases with suspected bone metastasis. We did not find any study in which patients with suspicious metastasis findings on scintigraphy were included as a separate group in survival analysis. It was observed that 30% of the patients who had suspicious findings for metastasis in bone scintigraphy died due to prostate cancer within the period specified for the study. The predicted mean survival time (71.1 ± 14.4 months) in the patient group with bone scintigraphy for suspected metastasis was a value between the predicted mean survival time of patients with and without bone metastasis (47.4 ± 5.4 and 159.1 ± 8.6 months, respectively) (Figure 1). Considering both mortality rates and survival times, it cannot be ignored that patients with true metastases may be included in the patient group considered suspicious for metastasis in bone scintigraphy. Therefore, patients whose bone scintigraphy is reported as suspicious for metastasis should not be interpreted as negative in terms of bone metastasis without further examination by clinicians.
In our study, the predicted mean survival time of patients with metastasis to the skeletal system on bone scintigraphy (47.4 ± 5.4 months) was significantly shorter than those without metastasis (159.1 ± 8.6 months) (p = 0.0001). There are studies in the literature investigating the prognostic effect of bone scintigraphy [13,15,18,20,24,29-31]. The only study that we could reach comparing the survival times of patients with metastases and without metastases in bone scintigraphy was the study of 128 patients published by Lund, et al. [15] in 1984. Although the survival times given in this study were shorter in the metastatic group, it was observed that the survival time of all patients did not exceed 60 months, regardless of bone metastasis. In our study, the survival of cases with suspected bone metastasis and cases without metastasis is longer than their cases. It may be probably due to changing treatment options, but since the prognostic effects of the treatment could not be studied in our study, it is not possible to explain the subject.
Contrary to studies [32,33] emphasizing that age is an important prognostic factor, age was not found as a strong prognostic factor in our study. Öbek, et al. [33] found that the recurrence rate in patients over 70 years of age was higher than those of 70 years and younger and reported that age was an independent prognostic factor in predicting recurrence after radical prostatectomy. Wyatt, et al. [33] also showed that age is an important prognostic factor in predicting survival time in androgen-independent patients. He, et al. [34] concluded in their study with prostate cancer patients in which they detected bone metastases that age is not an independent prognostic factor in predicting survival time. In our study, when patients were divided into two groups younger than 70 years old and over 70 years old, the distribution of patients in both groups was equal (134 and 131 patients, respectively). The statistical significance level was p = 0.0001 in subgroups of Gleason score, PSA level, and bone scintigraphy findings in the survival time analysis, while the statistical significance level was the lowest in age groups (p = 0.031). In the Kaplan-Meier analysis, a significant difference was found between the predicted mean survival time of patients under 70 years of age and patients over 70 years of age, while Cox regression analysis concluded that age is not an independent prognostic factor.
When the patients were evaluated according to the PSA level, it was found that the PSA level was < 10 ng/ml in 38% of 243 patients, and the PSA level was 10-20 ng/ml in 20%. In approaches that require bone scintigraphy considering the prostate-specific antigen level, the limit value chosen for PSA is usually 20 ng/ml [8,10-13]. Some approaches accept a cut-off value of 10 ng/ml [8,10,36]. In our study, patients with a PSA level of ≤ 20 ng/ml were as high as 58% (141 of 243 patients). We observed that the mean PSA values of the patients with metastasis on bone scintigraphy (285.7 ± 783.2 ng/ml) were found to be greater than the mean PSA values of the patients without metastasis (26.8 ± 42.8 ng/ml) and those with suspicious findings in terms of metastasis (30.2 ± 35.7 ng/ml). Rigaud, et al. [29] found the mean PSA values to be 975 ng/ml in their study with 86 patients who were found to have metastases on bone scintigraphy. In our study group, the number of patients with bone metastases with a Gleason score of > 7 (43% vs. 47%) and the mean age (71,2 vs. 71,3) were similar to theirs. The PSA averages of patients with bone metastasis in their study were significantly higher than in our study. The highest PSA value in their study (12420 ng/ml) was more than twice the highest PSA value in our study (5459 ng/ml). We think that this very high value raises the average.
When the average survival time was examined within the PSA subgroups, it was found that the mean life expectancy in the group with > 50 ng/ml was significantly shorter than in the other groups. When the life expectancy was examined according to the PSA level, it was found that the life expectancy of patients with > 20-50 ng/ml and a 10-20 ng/ml was slightly longer than that of patients with < 10 ng/ml. However, there was no statistically significant difference in terms of life span. In Kaplan-Meier survival analysis, the predicted mean survival time of patients with PSA 10 - 20 ng/ml (118.1 ± 14.8 months) was found to be slightly longer than the predicted mean survival time of patients with PSA < 10 ng/ml (106.9±4.2 months). The predicted mean survival time of patients with PSA > 20-50 ng/ml (87.6 ± 7.4 months) and patients with PSA > 50 ng/ml (51.7 ± 6.2 months) was shorter than the patients with PSA < 10 ng/ml and PSA 10-20ng/ml. The reason for this difference in the predicted mean survival time, which was not monitored in the graphical analysis may be that the patient with the lowest survival time (0.3 months) calculated in our study was in the PSA < 10 ng/ml group, and the patient with the second-highest life expectancy (152.8 months) was in PSA of 10-20 ng/ml group. In general, we found that the higher the PSA value, the shorter the predicted mean survival time. Abu-Hamar, et al. [35] found that the time to progression of patients with a PSA level of 50 ng/ml (32 months) was significantly longer than the time to progression of patients with a PSA level of > 50 ng/ml (24 months) (p = 0.002). Our findings are similar to theirs.
When the survival time was examined according to the Gleason score, it was seen that the more the Gleason score increased, the shorter the survival time (Figure 4). In our study, we saw that 41 (59%) of 70 patients with bone metastasis died due to prostate cancer. We calculated the predicted mean survival time as 47.4 ± 5.4 months. Yamashita, et al. [20], in their study with 76 prostate cancer patients with bone metastasis, found that 50 (66%) of these patients died due to prostate cancer. They calculated the median survival time to be 24.5 months (range of 3-100 months). The Gleason score of 60% of our patients with metastasis was above 7. They reported poor histological differentiation of the primary tumor in 37% of patients. Although our rate of patients with a Gleason score > 7 in our study was approximately twice as much as their study, our rate of patients who died due to prostate cancer was lower than theirs. When the patients were compared by age groups, it was found to be similar. The average age of our patients with bone metastasis was 71.2 years old, theirs was 68.7 years old. In the study of Yamashita, et al. [20], the PSA value comparison between the groups could not be evaluated, since there was no data about the serum PSA values of the patients before whole-body bone scintigraphy. Abu-Hamar, et al. [35], similar to our study, reported a shorter survival time in patients with a Gleason score above 7 (poorly differentiated). They reported that patients with a Gleason score ≤ 7 (33 months) had longer survival than patients with a Gleason score> 7 (24 months) (p < 0.001).
In the Cox regression analysis, it was seen that the most important factor independently affecting survival was bone scintigraphy findings (Wald = 19.7; p = 0.0001). Although PSA level and Gleason score were weaker, they were observed as independent prognostic factors. On the other hand, age was found to have no significant effect on prognosis. Abu-Hamar, et al. [35], in their study with 92 patients with metastatic prostate cancer, found similar to our study, the initial PSA level and Gleason score of the patient were useful markers in predicting prognosis. However, they did not find any statistical difference between the time to progression of the groups with > 6 and ≤ 6 bone areas in which metastasis was observed among these patients. He, et al. [34] showed that the Gleason score (p = 0.044) is an independent prognostic factor similar to our study, but besides that, unlike our study, they did not find a significant relationship between PSA level (p = 0.973) and survival time.
In our study, we found that the predicted survival time was shortened in patients with bone metastasis, high serum PSA levels, high Gleason score, and over 70 years of age. We concluded that bone metastasis detected in bone scintigraphy was the most important prognostic factor in predicting the survival time of patients, followed by Gleason score, serum PSA level, and age.
The limitations of this retrospective study, we do not have clear data on other prognostic factors such as the treatment status of the patients, familial prostate cancer data, PSA variants, and TNM classification. Only age, serum PSA level measured before the bone scintigraphy, Gleason score at the time of diagnosis, and presence of bone metastasis were used as prognostic factors.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019 Jan;69(1):7-34. doi: 10.3322/caac.21551. Epub 2019 Jan 8. PMID: 30620402.
- Güney Y, Yılmaz S, Türkcü Ü, Kurtman C. Kemik metastazlarında tanı ve tedavi. Acta Oncologica Turcica. 2008;41:1-6.
- Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, Fossati N, Gross T, Henry AM, Joniau S, Lam TB, Mason MD, Matveev VB, Moldovan PC, van den Bergh RCN, Van den Broeck T, van der Poel HG, van der Kwast TH, Rouvière O, Schoots IG, Wiegel T, Cornford P. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2017 Apr;71(4):618-629. doi: 10.1016/j.eururo.2016.08.003. Epub 2016 Aug 25. PMID: 27568654.
- Greene KL, Albertsen PC, Babaian RJ, Carter HB, Gann PH, Han M, Kuban DA, Sartor AO, Stanford JL, Zietman A, Carroll P; American Urological Association. Prostate specific antigen best practice statement: 2009 update. J Urol. 2013 Jan;189(1 Suppl):S2-S11. doi: 10.1016/j.juro.2012.11.014. PMID: 23234625.
- Hirobe M, Takahashi A, Hisasue S, Kitamura H, Kunishima Y, Masumori N, Iwasawa A, Fujimori K, Hasegawa T, Tsukamoto T. Bone scanning--who needs it among patients with newly diagnosed prostate cancer? Jpn J Clin Oncol. 2007 Oct;37(10):788-92. doi: 10.1093/jjco/hym097. Epub 2007 Oct 2. PMID: 17911377.
- Kosuda S, Yoshimura I, Aizawa T, Koizumi K, Akakura K, Kuyama J, Ichihara K, Yonese J, Koizumi M, Nakashima J, Fujii H. Can initial prostate specific antigen determinations eliminate the need for bone scans in patients with newly diagnosed prostate carcinoma? A multicenter retrospective study in Japan. Cancer. 2002 Feb 15;94(4):964-72. PMID: 11920464.
- Ayyathurai R, Mahapatra R, Rajasundaram R, Srinivasan V, Archard NP, Toussi H. A study on staging bone scans in newly diagnosed prostate cancer. Urol Int. 2006;76(3):209-12. doi: 10.1159/000091620. PMID: 16601380.
- Pal RP, Thiruudaian T, Khan MA. When is a bone scan study appropriate in asymptomatic men diagnosed with prostate cancer? Asian J Androl. 2008 Nov;10(6):890-5. doi: 10.1111/j.1745-7262.2008.00427.x. PMID: 18958353.
- Spencer JA, Chng WJ, Hudson E, Boon AP, Whelan P. Prostate specific antigen level and Gleason score in predicting the stage of newly diagnosed prostate cancer. Br J Radiol. 1998 Nov;71(851):1130-5. doi: 10.1259/bjr.71.851.10434906. PMID: 10434906.
- O'Sullivan JM, Norman AR, Cook GJ, Fisher C, Dearnaley DP. Broadening the criteria for avoiding staging bone scans in prostate cancer: a retrospective study of patients at the Royal Marsden Hospital. BJU Int. 2003 Nov;92(7):685-9. doi: 10.1046/j.1464-410x.2003.04480.x. PMID: 14616446.
- Tekdoğan ÜY, Ortapamuk H, Oğuz H, Başar MM, Naldöken S, Atan A. Prostat kanserli hastalarda prostat spesifik antijen (PSA) kemik metastazlarının belirleyicisi midir? Türk Üroloji Dergisi. 2001;27:433-436.
- Partin AW, Kattan MW, Subong ENP, Walsh PC, Wojno KJ, Oesterling JE, Scardino PT, Pearson JD. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. JAMA. 1997;277:1445-1451.
- Soloway MS, Hardeman SW, Hickey D, Raymond J, Todd B, Soloway S, Moinuddin M. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer. 1988 Jan 1;61(1):195-202. doi: 10.1002/1097-0142(19880101)61:1<195::aid-cncr2820610133>3.0.co;2-y. PMID: 3334948.
- Ishikawa S, Soloway MS, Van der Zwaag R, Todd B. Prognostic factors in survival free of progression after androgen deprivation therapy for treatment of prostate cancer. J Urol. 1989 May;141(5):1139-42. doi: 10.1016/s0022-5347(17)41193-1. PMID: 2651713.
- Lund F, Smith PH, Suciu S. Do bone scans predict prognosis in prostatic cancer? A report of the EORTC protocol 30762. Br J Urol. 1984 Feb;56(1):58-63. doi: 10.1111/j.1464-410x.1984.tb07165.x. PMID: 6697108.
- Lund F, Smith PH, Suciu S. Do bone scans predict prognosis in prostatic cancer? A report of the EORTC protocol 30762. Br J Urol. 1984 Feb;56(1):58-63. doi: 10.1111/j.1464-410x.1984.tb07165.x. PMID: 6697108.
- Walz J, Haese A, Scattoni V, Steuber T, Chun FK, Briganti A, Montorsi F, Graefen M, Huland H, Karakiewicz PI. Percent free prostate-specific antigen (PSA) is an accurate predictor of prostate cancer risk in men with serum PSA 2.5 ng/mL and lower. Cancer. 2008 Nov 15;113(10):2695-703. doi: 10.1002/cncr.23885. PMID: 18853417.
- Pollen JJ, Gerber K, Ashburn WL, Schmidt JD. Nuclear bone imaging in metastatic cancer of the prostate. Cancer. 1981 Jun 1;47(11):2585-94. doi: 10.1002/1097-0142(19810601)47:11<2585::aid-cncr2820471113>3.0.co;2-u. PMID: 6266636.
- Hamdy FC. Prognostic and predictive factors in prostate cancer. Cancer Treat Rev. 2001 Jun;27(3):143-51. doi: 10.1053/ctrv.2000.0208. PMID: 11417964.
- Yamashita K, Denno K, Ueda T, Komatsubara Y, Kotake T, Usami M, Maeda O, Nakano S, Hasegawa Y. Prognostic significance of bone metastases in patients with metastatic prostate cancer. Cancer. 1993 Feb 15;71(4):1297-302. doi: 10.1002/1097-0142(19930215)71:4<1297::aid-cncr2820710421>3.0.co;2-s. PMID: 8435807.
- Tanaka N, Fujimoto K, Shinkai T, Nakai Y, Kuwada M, Anai S, Miyake M, Hirayama A, Hasegawa M, Hirao Y. Bone scan can be spared in asymptomatic prostate cancer patients with PSA of <=20 ng/ml and Gleason score of <=6 at the initial stage of diagnosis. Jpn J Clin Oncol. 2011 Oct;41(10):1209-13. doi: 10.1093/jjco/hyr118. Epub 2011 Aug 23. PMID: 21862505.
- Zaman MU, Fatima N, Sajjad Z. Metastasis on bone scan with low prostate specific antigen (≤20 ng/ml) and Gleason's score (<8) in newly diagnosed Pakistani males with prostate cancer: should we follow Western guidelines? Asian Pac J Cancer Prev. 2011;12(6):1529-32. PMID: 22126493.
- Lai MH, Luk WH, Chan JC. Predicting bone scan findings using sPSA in patients newly diagnosed of prostate cancer: feasibility in Asian population. Urol Oncol. 2011 May-Jun;29(3):275-9. doi: 10.1016/j.urolonc.2009.05.007. Epub 2009 Sep 6. PMID: 19734069.
- Ishizuka O, Tanabe T, Nakayama T, Kawakami M, Kinebuchi Y, Nishizawa O. Prostate-specific antigen, Gleason sum and clinical T stage for predicting the need for radionuclide bone scan for prostate cancer patients in Japan. Int J Urol. 2005 Aug;12(8):728-32. doi: 10.1111/j.1442-2042.2005.01118.x. PMID: 16174046.
- Al-Ghazo MA, Ghalayini IF, Al-Azab RS, Bani-Hani I, Barham A, Haddad Y. Do all patients with newly diagnosed prostate cancer need staging radionuclide bone scan? A retrospective study. Int Braz J Urol. 2010 Nov-Dec;36(6):685-91; discussion 691-2. doi: 10.1590/s1677-55382010000600006. PMID: 21176275.
- Lee SH, Chung MS, Park KK, Yom CD, Lee DH, Chung BH. Is it suitable to eliminate bone scan for prostate cancer patients with PSA ≤ 20 ng/mL? World J Urol. 2012 Apr;30(2):265-9. doi: 10.1007/s00345-011-0728-6. Epub 2011 Jul 16. PMID: 21779835; PMCID: PMC3321272.
- Salonia A, Gallina A, Camerota TC, Picchio M, Freschi M, DaPozzo LF, Guazzoni G, Fazio F, Rigatti P, Montorsi F. Bone metastases are infrequent in patients with newly diagnosed prostate cancer: analysis of their clinical and pathologic features. Urology. 2006 Aug;68(2):362-6. doi: 10.1016/j.urology.2006.02.009. PMID: 16904453.
- Ritenour CW, Abbott JT, Goodman M, Alazraki N, Marshall FF, Issa MM. The utilization of Gleason grade as the primary criterion for ordering nuclear bone scan in newly diagnosed prostate cancer patients. ScientificWorldJournal. 2009 Oct 2;9:1040-5. doi: 10.1100/tsw.2009.113. PMID: 19802499; PMCID: PMC5823094.
- Rigaud J, Tiguert R, Le Normand L, Karam G, Glemain P, Buzelin JM, Bouchot O. Prognostic value of bone scan in patients with metastatic prostate cancer treated initially with androgen deprivation therapy. J Urol. 2002 Oct;168(4 Pt 1):1423-6. doi: 10.1097/01.ju.0000030900.55714.76. PMID: 12352409.
- Knudson G, Grinis G, Lopez-Majano V, Sansi P, Targonski P, Rubenstein M, Sharifi R, Guinan P. Bone scan as a stratification variable in advanced prostate cancer. Cancer. 1991 Jul 15;68(2):316-20. doi: 10.1002/1097-0142(19910715)68:2<316::aid-cncr2820680218>3.0.co;2-0. PMID: 2070330.
- Ohmori K, Matsui H, Yasuda T, Kanamori M, Yudoh K, Seto H, Tsuji H. Evaluation of the prognosis of cancer patients with metastatic bone tumors based on serial bone scintigrams. Jpn J Clin Oncol. 1997 Aug;27(4):263-7. doi: 10.1093/jjco/27.4.263. PMID: 9379516.
- Wyatt RB, Sánchez-Ortiz RF, Wood CG, Ramirez E, Logothetis C, Pettaway CA. Prognostic factors for survival among Caucasian, African-American and Hispanic men with androgen-independent prostate cancer. J Natl Med Assoc. 2004 Dec;96(12):1587-93. PMID: 15622688; PMCID: PMC2568655.
- Obek C, Lai S, Sadek S, Civantos F, Soloway MS. Age as a prognostic factor for disease recurrence after radical prostatectomy. Urology. 1999 Sep;54(3):533-8. doi: 10.1016/s0090-4295(99)00168-5. PMID: 10475367.
- He J, Zeng ZC, Yang P, Chen B, Jiang W, Du SS. Clinical features and prognostic factors for patients with bone metastases from prostate cancer. Asian J Androl. 2012 May;14(3):505-8. doi: 10.1038/aja.2012.24. Epub 2012 Apr 16. PMID: 22504872; PMCID: PMC3720166.
- Abu-Hamar Ael H, Gameel TA. Prognostic Significance of PSA, Gleason Score, Bone Metastases in Patients with Metastatic Prostate Cancer Under Palliative Androgen Deprivation Treatment. J Egypt Natl Canc Inst. 2009 Sep;21(3):229-36. PMID: 21132033