More Information
Submitted: July 01, 2022 | Approved: July 14, 2022 | Published: July 15, 2022
How to cite this article: Tütüncü M, Özşengezer SK, Karakayali T, Altun ZS. The effects of boric acid and disodium pentaborate dechydrate in metastatic prostate cancer cells. J Radiol Oncol. 2022; 6: 012-017.
DOI: 10.29328/journal.jro.1001041
Copyright License: © 2022 Tütüncü M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The effects of boric acid and disodium pentaborate dechydrate in metastatic prostate cancer cells
Merve Tütüncü *, Selen Kum Özşengezer, Tuğba Karakayali and Zekiye S Altun
*, Selen Kum Özşengezer, Tuğba Karakayali and Zekiye S Altun
Department of Basic Oncology, Dokuz Eylul University, Institute of Oncology, Izmir, Turkey
*Address for Correspondence: Merve Tütüncü, Department of Basic Oncology, Dokuz Eylul University Institute of Oncology, Izmir, Turkey, Email: [email protected]
Boron and their derived molecules have prevention or treatment potential against prostate cancer. In this study, we aim to investigate the effects of Boric acid (BA) and Disodium Pentaborate Dechydrate (DPD) in metastatic prostate cancer cells such as DU-145 which is brain metastatic prostate cancer, and PC3 which is bone metastatic prostate cancer.
Metastatic human prostate cancer cell lines, PC-3 and DU-145, were used to show whether inhibition effects of BA and DPD on prostate cancer cells in this study. BA and DPD were applied for 24 hours to the cells. Cell viability determination was performed using WST-1 assay. Apoptotic cell death was evaluated with Annexin-V/PI flow cytometric analysis and caspase-3 expression immunohistochemically. A wound healing assay was also used to measure cancer cell migration after exposure to BA and DPD.
Applying BA and DPD made inhibition of cell proliferation in both BA (1 mM) and DPD (7 mM) at 24 h. The results of Annexin-V/PI showed that DPD induced higher levels of apoptosis than BA in both prostate cancer cells. Caspase-3 expressions were also higher than BA with DPD in both metastatic prostate cancer cells. We evaluated cell migration using a wound healing assay and the result showed that cell migration was inhibited with BA and DPD in both cells.
Both BA and DPD inhibited the cell viability of metastatic prostate cancer cells. Apoptotic cell death with applying DPP had a higher rate than BA treatment. Moreover, BA and DPD inhibited cell migration in both cells when we compared them with control. This study’s results showed that BA and DPD of boron derivates significantly induced cells to apoptosis and the migration was inhibited by the derived form of boron in metastatic prostate cancer cells.
Prostate cancer is the second most common cancer type in men with high incidence and mortality rates. In addition, prostate cancer is one of the most important cancer types and causes of death in malignant tumors in men [1]. Different treatment options have been used especially for each case with the various stages of patients with prostate cancer [2]. However, Prostate cancer treatments such as chemotherapy or radiotherapy can seriously affect a patient’s quality of life. The anti-proliferative effect of boron derivatives by reducing cancer cell viability suggests that they could be a promising new strategy for the treatment of advanced prostate cancer.
Boron is a trace element for all organisms, also humans. It has got a high affinity for oxygen that’s important for balancing the ROS [3]. Soluble forms include boric acid B(OH)3 and the monovalent anion B(OH)4- with the presence of the dominant form dependent upon solvent pH. In plasma, boric acid predominates, and its concentration reflects dietary intake and respiratory exposure [4]. Thanks to its antioxidant or anticancer properties, it takes part in many physiological and biochemical processes [5,6]. Treatment of nude mice injected with androgen-sensitive LNCaP prostate cancer cells with boric acid caused a decrease in tumor growth and inhibition of the enzymatic activity of the prostate-specific antigen [7]. In the cell culture method, researchers found that boron compounds show growth inhibitory effects [7]. However, Mehmet, et al. showed in their study that DPD has a better anti-cancer potential as an inhibitor of cancer cell viability than boric acid [8].
Apoptosis is a type of programmed cell death that results in the orderly and efficient elimination of injured cells, along with those due to DNA damage or in the course of development. Apoptosis can develop due to intracellular or extracellular signal pathways [9]. Normally, most chemotherapeutic agents induce cancer cell death via apoptosis. Caspase-3 is an effector caspase in the apoptotic pathway so the determination of caspase-3 activation is the most important step for the completion of apoptotic cell death.
Our study, it was aimed to investigate the effects of BA and DPD, which are boron derivatives, on the viability and induction of apoptosis in DU-145 and PC-3 advanced metastatic prostate cancer cell lines and we also showed that inhibition of cell migration by BA and DPD was also determined.
This study was carried out in Dokuz Eylul University Oncology Institute Basic Oncology Department Laboratories. Advanced stage metastatic human prostate cancer cell lines, DU-145 (ATCC) and PC-3 (ATCC) were genteelly obtained from Prof. Dr. Kemal Sami Korkmaz, Ege University Faculty of Engineering Bioengineering Department. Boric acid (BA) and Disodium Pentaborate Decahydrate (DPD) were also kindly provided by Prof. Dr. Mehmet Korkmaz, Celal Bayar University. All conditions were replicated at least by three times.
DU-145 and PC-3 prostate cancer cell culture
Cells were grown using complete media created by adding 1% L-glutamine and 1% penicillin-streptomycin and 10% fetal bovine serum to RPMI-1640 medium at 37°C 5% CO2 humidified incubator conditions. BA (250 µM - 1 mM) [10] and DPD (7 mM) [8] agents were added to the DU-145 and PC-3 cell lines and the cytotoxic effect of the agents was evaluated. The cells were passaged when sufficient confluence is achieved. Boron derivatives were freshly prepared from stock solutions. The main stock solution was prepared by dissolving boric acid (BA; 1 mM) and disodium pentaborate decahydrate (DPD; 7 mM) in distilled water.
WST-1 cell proliferation
Cell proliferation assay was performed in accordance with WST-1 kit (Roche) protocol [8]. Cells were seeded in 96-well cell culture plates at 1 × 104 in each well and checked the next day. After the BA and DPD were applied in the cells by 24 h incubations, 10 μl WST-1 solution for each 100 μl was added to these cells. Control cells were incubated under the same conditions without boron derivatives. After incubation periods, the absorbances of the cells were measured test wavelength of 450 nm and the reference wavelength at 630 nm was measured with an ELISA plate reader (Thermo). The control group was accepted as 100% viable and compared with the BA and DPD-induced changes. The half-maximal lethal concentrations (LD50) of the agents were calculated according to to control cell viability.
Apoptotic cell death analysis
In our study, Annexin-V/PI Assay (Biovision) was used to determine apoptosis cells. Annexin-V can be labeled with FITC, a fluorescent substance, which can bind to the phosphatidylserine on the outer surface of the cell [11]. Thus, the apoptotic cells to which FITC is conjugated with annexin-V can be made visible. The principle of the method is based on of the extent apoptosis determination using flow cytometry of cells stained with annexin-V-FITC and PI. Briefly, cells were washed three times with cold PBS and binding buffer was added to 1×105 cells/mL cell to be 100 µl and the cells were re-suspended. After the Annexin V-FITC and PI were added, the cells were transferred to a tube. After mixing, they were kept at room temperature for 15 minutes. Analyses were carried out using a flow cytometer (Accuri, BD) at 488 nm excitation and 530 nm emission wavelengths for Annexin-V and 480 nm excitation and 588 nm emission wavelengths for PI, and then assessed using the BD Accuri C6 Software (BD Biosciences).
Caspase-3 expression analysis by immunohistochemical analysis (IHC)
The grown DU-145 and PC-3 cells are removed from the flask by using a cell scraper and taken into lysine and positively charged slides and spread on the surface by dripping [12]. Firstly, the slides were incubated at 37 oC for overnight to dry of slides. Before starting the staining process, slides were kept in a 4% formaldehyde solution for 1 hour, after being dried and fixed, the slides were exposed to 0.1% Tween 20 for 2 minutes and washed with distilled water 3 times. 1:200 Dilution of active-caspase 3 antibodies (Bioss), and Rabbit Host Seconder Antibody (Roche) was used to examine the immunohistochemical analysis of DU-145 and PC-3 prostate cancer cells. After this procedure, the slides were kept in xylol for 1 hour before mounting with Stellan. All positive cells were counted via a light microscope (Olympus, Germany).
Wound healing analyzing for cell migration
3 × 105 cells were seeded to each well of the 6-well plate for each condition and incubated for 24 hours. After cell confluency, a wound was made (with the help of a 20 µL micropipette). Then, the cells were washed with a medium and removed from the suspension. After this, the medium with BA and DPD was added to the cells. The control sample contained a standard medium with 1% FBS in the cells. The gap areas of all conditions were taken the following every 6 hours (0 h, 6 h, and 12 h) and recorded the results, after the experimental process, the gaps were measured by Image-J.
Statistical analysis
Data were presented as mean ± standard deviation. All statistical analyses were made using the SPSS 22.0 software program (BMI, USA). Differences were compared with the Mann-Whitney U test. The values p < 0.05 was considered statistically significant.
In our study, we investigated the 24-hour effect of BA and DPD on human-derived metastatic DU-145 and PC-3 prostate cancer cell lines. We evaluated cell viability by WST-1 test, apoptotic cell death with flow cytometric Annexin-V/PI dye, scratch wound model for migration analysis, and immunohistochemistry with anti-caspase 3 antibody.
WST-1 viability results
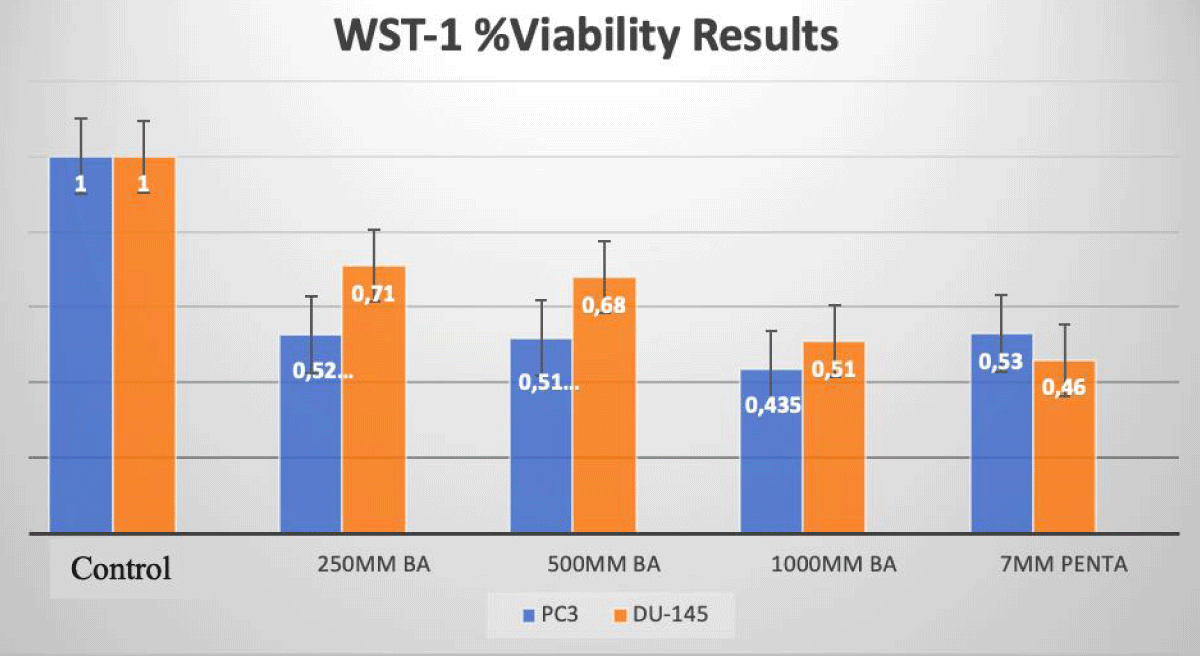
We evaluated viability with disodium pentaborate decahydrate (7 mM) and boric acid (0, 250 µM, 500 µM, 1 mM) for DU-145 and PC-3 cells at 24 h incubation (Figure 1).
In PC-3 prostate cancer cells, the cell viability decreased at a rate of 57%, and 61% with treatment with DPD (7 mM) and BA (1 mM) when compared to the control group after 24 h of incubation, respectively (p < 0.05; Figure 1). In DU-145 prostate cancer cells, the cell viability decreased at a rate of 50%, and 58% with the treatment of DPD (7 mM) and BA (1 mM), when compared to the control group after 24 h of incubation, respectively (p < 0.05; Figure 1).
Figure 1: WST-1 Results of Cell Lines. The effects of BA and DPD on PC-3 and DU-145 cell viability were assessed by the WST-1 assay for 24 h. In PC-3 cells, IC50 dose of DPD (7 mM) and BA (1mM) treatment has resulted in a decrease at the rate of 57% and 61% for 24 h when compared to control, respectively (p < 0.05). In DU-145 cells, IC50 dose of DPD (7 mM) and BA (1 mM) treatment has resulted in a decrease at the rate of 50% and 58% for 24 h when compared to control, respectively (p < 0.05). *Statistically significant from control exposures (p < 0.05).
Apoptotic cell death analysis by flow cytometry
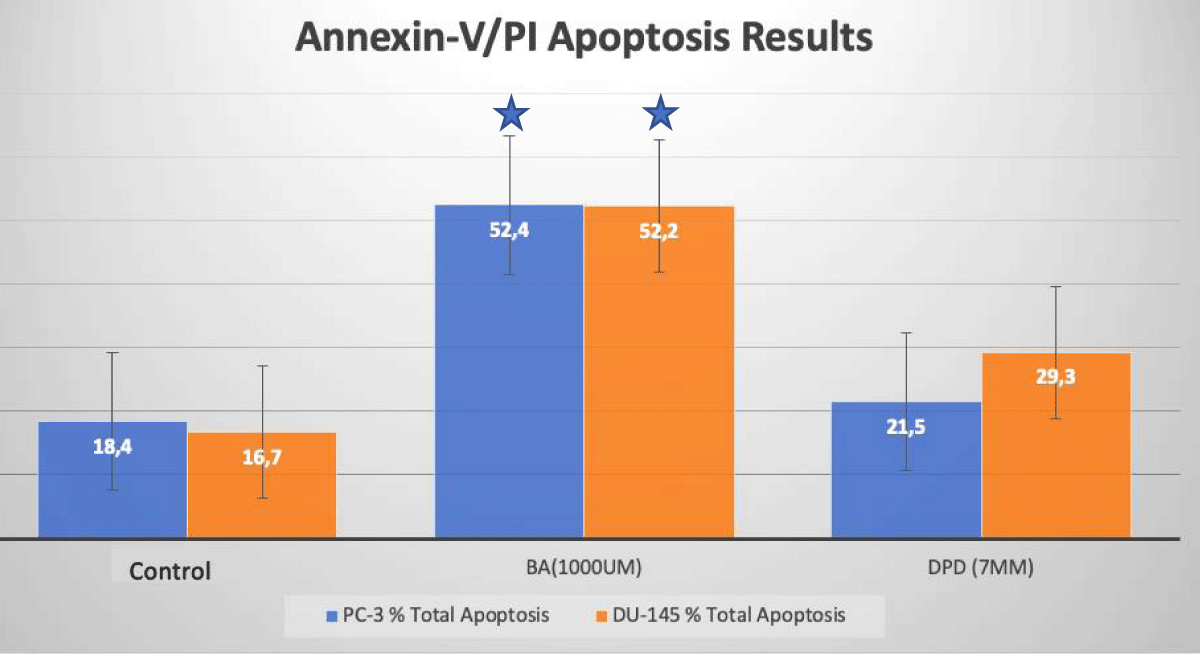
Apoptotic cell death was determined by Annexin-V/PI Flow Cytometry analysis after 24 hours of incubations of agents. The results obtained from the analysis are given in Figure 2. In PC-3 prostate cancer cells, apoptosis increased at a rate of 52.4%, and 21.5% with treatment with DPD (7 mM) and BA (1 mM) when compared to the control group after 24 h of incubation, respectively (p < 0.05; Figure 2). In DU-145 prostate cancer cells, the apoptosis increased at a rate of 52.4%, and 29.3% with treatment with DPD (7 mM) and BA (1 mM), when compared to the control group after 24 h of incubation, respectively (p < 0.05; Figure 2).
Figure 2: Apoptotic Cell Death Results. Apoptotic cell death analysis of BA and DPD-treated PC-3 and DU-145 cells by Annexin-V/PI flow-cytometry. In PC-3 and DU-145 cells, DPD (7 mM) and BA (1 mM) at 24 h incubations induced apoptosis when compared to control. *Statistically significant from control exposures (p < 0.05).
Evaluation of the apoptotic cell death of active-Caspase-3 expression
Active-Caspase-3 protein expression was defined by the immunohistochemical method. All groups including DU-145 and PC-3 cells were stained with the active-Caspase-3 primer antibody. After the staining procedure, cells were counted and active-caspase3 positive cells were determined by light microscopy.
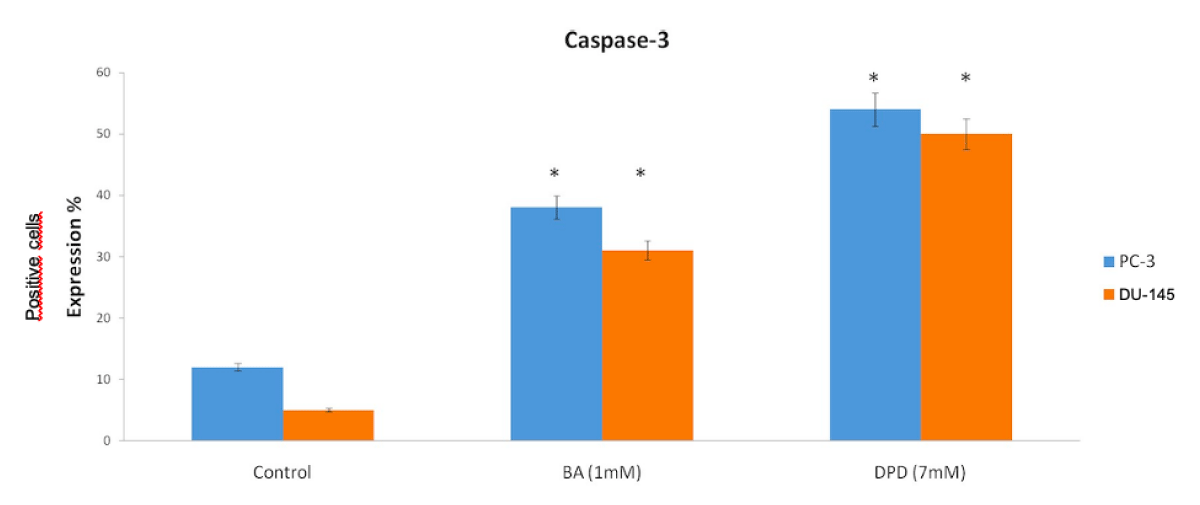
The Control group of DU-145 cells’ caspase-3 positive cells were counted and the result showed that the control group had 5% apoptotic cells (Figures 3,4).
Figure 3: Anti-Caspase3 Expression Scores in Du-145 and PC3 Cells. Caspase-3 protein expression immunohistochemistry scores in PC-3 and DU-145 cell line. Differential expression of caspase-3 in PC-3 and DU-145 cells was significant compared to control. When the anti-caspase3 positive cells were counted, in PC-3 and DU-145 cells, caspase-3 expression was significantly increased with treatment of BA and DPD cells (p < 0.05). *Statistically significant from control exposures (p < 0.05).
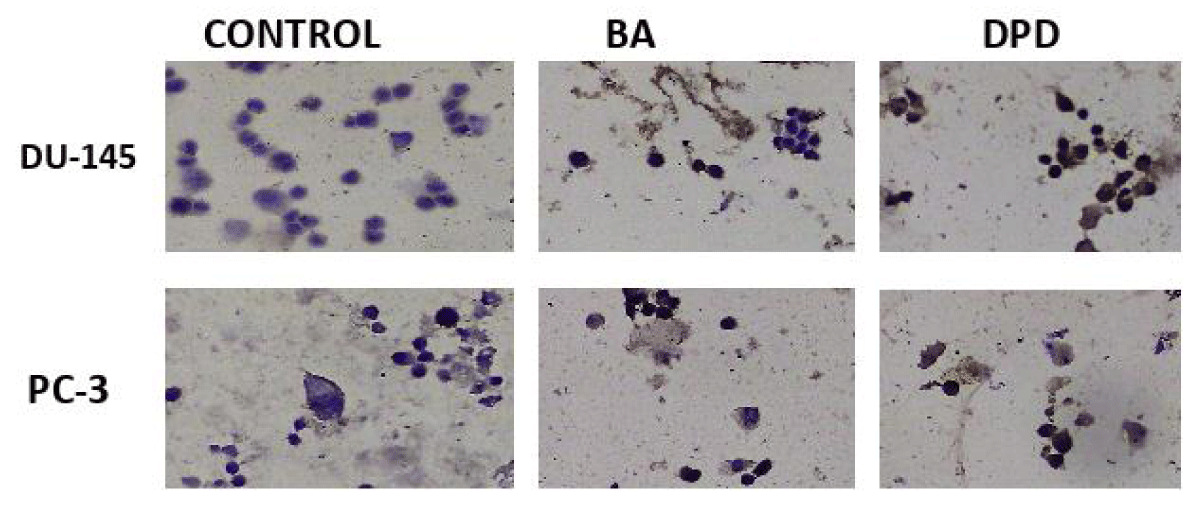
Figure 4: Caspase-3 protein expression results in PC-3 and DU-145 cells 20x.
The other groups’ positive cells were 31% and 50% with BA and DPD, respectively (p < 0.05, Figures 3 4).
PC-3 cells also were evaluated by staining with active-caspase-3 primer antibody after 24h of Boric Acid and Disodium Pentaborate Dechydrate applied. After this, active-caspase 3 positive cells indicated apoptosis with 12%, 38%, and 54% positive with Control, BA, and DPD in PC-3 cells (p <0.05, Figure 3,4).
Wound healing assay results for cancer cell migration
3 × 105 cells were seeded in 6-well plates and incubated for 24 h. A wound model was created in the confluent cell plate, and a complete medium with 1% FBS and the agents were applied to the cells. The images of the wound healing assay were taken each 6 hours; before the agent’s application, a sixth hour later, and at the end of the 12th hour (0 h, 6 h and 12 h). The results are seen in Figure 5 and Figure 6. Depending on the time in DU-145 and PC-3 cell lines, agent administration was found to inhibit cell migration when compared to control. Inhibition of the cell migration showed a time-dependent trend in Figures 5,6.
Figure 5: Wound Healing Model of DU-145 cells. Wound Healing Model of DU-145 cells. Cells were seeded and incubated overnight; cells were applied with BA (1 mM) and DPD (7 mM). Cell migration was monitored under a phase-contrast microscope every 6 hours for 12 hours. Data are representative of the results that agents inhibited migration in DU-145 cells. Shows images taken at 0 h, 6 h and 12 h. The black line shows the wound.
Figure 6: Wound Healing Model of PC-3 cells. Wound Healing Model of PC-3 cells. Cells were seeded and incubated overnight; cells were treated with BA (1 mM) and DPD (7 mM). Cell migration was monitored under a phase-contrast microscope every 6 hours for 12 hours. Data are representative of the results that agents inhibited migration in PC-3 cells. Shows images taken at 0 h, 6 h and 12 h. The black line shows the wound.
Derivatives of Boron have been shown associated with both preventive and anti-carcinogenic effects in prostate cancer and some other cancers [3,6-8]. Some epidemiologic studies showed that derives of boron intake could inhibit prostate cancer [8,15]. With all this information, in our study, we aim to compare two boron derivatives including BA and DPD, on two types of advanced metastatic prostate cancer cells.
Henderson, et al. showed that boric acid significantly induced apoptosis based on inducing ER stress in a dose depending [10]. One study demonstrated that BA inhibits cell proliferation in both DU-145 (non-androgen sensitive) and LNCAP (androgen sensitive) prostate cancer cells depending on the dose. They also reported that both healthy prostate epithelial cell lines of RWPE-1 and PWR-1E were inhibited by BA, but they declared that these doses are above the physiological blood level in individuals [14]. They indicated that the result of applying boric acid to DU-145 cells showed an aging-like effect and boric acid reduced the rate of metastasis to make inhibition F-actin activation [10]. Barranco, et al. showed in their study, that boric acid improves the antiproliferative effect of chemopreventative agents and rising groundwater boron concentration decreases prostate cancer morbidity and mortality [15]. In this study, BA and DPD effects on both DU-145 and PC-3 cells were detected, BA and DPD inhibited cell viability at almost the same ratios in both DU-145 and PC-3 cells and our results are compatible with the literature, 24-hour administration of BA and DPD significantly reduced cell proliferation in both cells [8,16].
Moreover, our study also assessed apoptotic cell death through Annexin-V/PI staining and active caspase-3 protein expression by IHC. BA and DPD applied groups’ cell apoptotic cell ratios were significantly increased when we compared them with control. Compatible with these results, the caspase-3 expression was also increased in BA and DPD groups when we compared it to the control groups in both PC-3 and DU-145 cells.
Our results were observed in correlation with Henderson’s results, in which boron intake was shown to induce apoptosis. Henderson’s study showed the apoptosis effect because of ER stress by activating eIF2 alpha/ATF4 pathways in DU-145 brain metastatic prostate cell line [13]. Yamada, et al. examined the elF2 alpha/ATF4 pathways with the BA relationship. They suggested that the relationship of elF2 alpha/ATF4 pathways and BA depends on increasing antioxidant response element (ARE) genes; including heme oxygenase-1 (HMOX-1), NAD(P)H dehydrogenase quinone-1 (NQO1), and glutamate-cysteine ligase catalytic subunit (GCLC) [16].
Gallardo-Williams’ study reported that nutritional supplements with BA can inhibit PSA levels and reduce the development of tumor formation and proliferation of prostate cancer cells in xenograft models [7].
Migration plays a critical role in invasive and metastatic tumor formation. For this reason, revealing the effects of agents on migration is also important for potential treatment approaches. McAuley, et al. showed that BA treatment significantly reduces migration in metastatic prostate cancer cell lines but not in the healthy prostate epithelial cell [17]. In addition, according to our wound healing model results, BA and DPD decreased metastatic cancer cell invasions in both metastatic cell lines. McAully, et al. also showed similar results to our study. They also indicated that BA reduced the expression of RhoA, Rac1, and Cdc42 genes in DU-145 prostate cancer cells but not in prostate epithelial cells (RWPE-1) [17]. Korkmaz, et al. studied the DPD effect on the DU-145 cell line at different times. Compatible with this, DPD at 7mM showed induction of apoptosis in DU-145 prostate cancer cells in our study [8].
In our study, the result of applying BA and DPD showed inhibition of cell proliferation and decreasing cell invasion in both DU-145 and PC-3 cell lines. In addition, this study indicated the apoptotic effects of BA and DPD against metastatic prostate cancer cells through active caspase-3 protein expression. Our results suggested that DPD was more effective in terms of the anticancer field between boron derivatives (BA and DPD) for metastatic prostate cancer therapy. In the light of these results, we will gain more knowledge on the study models about metastatic prostate cancer and further evaluation of BA and DPD as effective chemotherapeutic agent candidates for prostate cancer treatment.
- Karantanos T, Corn PG, Thompson TC. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene. 2013 Dec 5;32(49):5501-11. doi: 10.1038/onc.2013.206. Epub 2013 Jun 10. PMID: 23752182; PMCID: PMC3908870.
- Abate-Shen C, Shen MM. Molecular genetics of prostate cancer. Genes Dev. 2000 Oct 1;14(19):2410-34. doi: 10.1101/gad.819500. PMID: 11018010.
- Devirian TA, Volpe SL. The physiological effects of dietary boron. Crit Rev Food Sci Nutr. 2003;43(2):219-31. doi: 10.1080/10408690390826491. PMID: 12705642.
- Woods WG. An introduction to boron: history, sources, uses, and chemistry. Environ Health Perspect. 1994 Nov;102 Suppl 7(Suppl 7):5-11. doi: 10.1289/ehp.94102s75. PMID: 7889881; PMCID: PMC1566642.
- Barranco WT, Kim DH, Stella SL Jr, Eckhert CD. Boric acid inhibits stored Ca2+ release in DU-145 prostate cancer cells. Cell Biol Toxicol. 2009 Aug;25(4):309-20. doi: 10.1007/s10565-008-9085-7. Epub 2008 May 31. PMID: 18516691.
- Nielsen FH. Biochemical and physiologic consequences of boron deprivation in humans. Environ Health Perspect. 1994 Nov;102 Suppl 7(Suppl 7):59-63. doi: 10.1289/ehp.94102s759. PMID: 7889883; PMCID: PMC1566640.
- Gallardo-Williams MT, Chapin RE, King PE, Moser GJ, Goldsworthy TL, Morrison JP, Maronpot RR. Boron supplementation inhibits the growth and local expression of IGF-1 in human prostate adenocarcinoma (LNCaP) tumors in nude mice. Toxicol Pathol. 2004 Jan-Feb;32(1):73-8. doi: 10.1080/01926230490260899. PMID: 14713551.
- Korkmaz M, Avcı CB, Gunduz C, Aygunes D, Erbaykent-Tepedelen B. Disodium pentaborate decahydrate (DPD) induced apoptosis by decreasing hTERT enzyme activity and disrupting F-actin organization of prostate cancer cells. Tumour Biol. 2014 Feb;35(2):1531-8. doi: 10.1007/s13277-013-1212-2. PMID: 24122279.
- Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011 Nov 11;147(4):742-58. doi: 10.1016/j.cell.2011.10.033. Erratum in: Cell. 2011 Dec 23;147(7):1640. PMID: 22078876; PMCID: PMC4511103.
- Barranco WT, Eckhert CD. Cellular changes in boric acid-treated DU-145 prostate cancer cells. Br J Cancer. 2006 Mar 27;94(6):884-90. doi: 10.1038/sj.bjc.6603009. PMID: 16495920; PMCID: PMC3216419.
- Altun ZS, Tanrıverdi Akhisaroğlu S, Batu J, Ates H, Giray H, Kocturk S. Discrimination Effectiveness of CK18 on Cell Death Modes in Colon Cancer Cells. Turk J Biochem. 2010; 35(1): 20–28.
- Javois LC. Immunocytochemistry Methods and Protocols. 2nd edition, Human Press. 64- 70.
- Barranco WT, Hudak PF, Eckhert CD. Evaluation of ecological and in vitro effects of boron on prostate cancer risk (United States). Cancer Causes Control. 2007 Feb;18(1):71-7. doi: 10.1007/s10552-006-0077-8. Erratum in: Cancer Causes Control. 2007 Jun;18(5):583-4. PMID: 17186423.
- Barranco WT, Eckhert CD. Boric acid inhibits human prostate cancer cell proliferation. Cancer Lett. 2004 Dec 8;216(1):21-9. doi: 10.1016/j.canlet.2004.06.001. PMID: 15500945.
- Yamada KE, Eckhert CD. Boric Acid Activation of eIF2α and Nrf2 Is PERK Dependent: a Mechanism that Explains How Boron Prevents DNA Damage and Enhances Antioxidant Status. Biol Trace Elem Res. 2019 Mar;188(1):2-10. doi: 10.1007/s12011-018-1498-4. Epub 2018 Sep 8. PMID: 30196486.
- Henderson KA, Kobylewski SE, Yamada KE, Eckhert CD. Boric acid induces cytoplasmic stress granule formation, eIF2α phosphorylation, and ATF4 in prostate DU-145 cells. Biometals. 2015 Feb;28(1):133-41. doi: 10.1007/s10534-014-9809-5. Epub 2014 Nov 26. PMID: 25425213; PMCID: PMC4300416.
- McAuley EM, Bradke TA, Plopper GE. Phenylboronic acid is a more potent inhibitor than boric acid of key signaling networks involved in cancer cell migration. Cell Adh Migr. 2011 Sep-Oct;5(5):382-6. doi: 10.4161/cam.5.5.18162. PMID: 21975546; PMCID: PMC3218604.