More Information
Submitted: December 12, 2022 | Approved: December 19, 2022 | Published: December 20, 2022
How to cite this article: Haidar ZS. Radiation-induced salivary gland damage/dysfunction in head and neck cancer: Nano-bioengineering strategies and artificial intelligence for prevention, therapy and reparation. J Radiol Oncol. 2022; 6: 027-044.
DOI: 10.29328/journal.jro.1001044
Copyright License: © 2022 Haidar ZS. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Artificial intelligence; Bioengineering; Biomimicry; Chemo-radio therapy; Irradiation; Radioprotection; Salivary gland; Xerostomia; Head and neck cancer; Oro-dental health
Radiation-induced salivary gland damage/dysfunction in head and neck cancer: Nano-bioengineering strategies and artificial intelligence for prevention, therapy and reparation
Ziyad S Haidar1-4*
1BioMAT’X I+D+I (HAiDAR LAB), University of the Andes, Santiago, Chile
2Center for Biomedical Research and Innovation (CiiB), University of the Andes, Santiago, Chile
3Doctoral Program in Biomedicine, Faculty of Medicine, University of the Andes, Santiago, Chile
4Department of Biomaterials and BioEngineering, Faculty of Dentistry, University of the Andes, Santiago, Chile
*Address for Correspondence: Prof. Dr. Ziyad S. Haidar. DDS, Implantologist (Cert Implantol), Oral and Maxillofacial Surgeon (MSc OMFS), FRSC (Canada), FICD, FICS, MBA, Ph.D. Professor and Scientific Director, Faculty of Dentistry, Universidad de los Andes, Santiago de Chile. Founder and Head/Director of BioMAT’X I+D+i (HAiDAR R&D&I) Research Group and Laboratory, (Laboratorio de Biomaterials, Farmacéuticos y Bioingeniería de Tejidos Cráneo Máxilo-Facial), Biomedical Research and Innovation Center / Centro de Investigación e Innovación Biomédica (CiiB), Faculty of Medicine, Department for Research, Development and Innovation, Universidad de los Andes, Avenida Mons. Álvaro del Portillo 12.455 - Las Condes, Santiago de Chile, Websites: https://www.uandes.cl/personas/ziyad-s-haidar/ and HAiDAR “BioMAT’X I+D+i” LAB: https://www.uandes.cl/biomatx-laboratorio-de-Biomaterials-farmaceuticos-y-bioingenieria-de-tejidos-craneo-maxilo-facial/ E-mail: [email protected]
Saliva is produced by and secreted from salivary glands. It is an extra-cellular fluid, 98% water, plus electrolytes, mucus, white blood cells, epithelial cells, enzymes, and anti-microbial agents. Saliva serves a critical role in the maintenance of oral, dental, and general health and well-being. Hence, alteration(s) in the amount/quantity and/or quality of secreted saliva may induce the development of several oro-dental variations, thereby the negatively-impacting overall quality of life. Diverse factors may affect the process of saliva production and quantity/quality of secretion, including medications, systemic or local pathologies and/or reversible/irreversible damage. Herein, chemo- and/or radio-therapy, particularly, in cases of head and neck cancer, for example, are well-documented to induce serious damage and dysfunction to the radio-sensitive salivary gland tissue, resulting in hypo-salivation, xerostomia (dry mouth) as well as numerous other adverse Intra-/extra-oral, medical and quality-of-life issues. Indeed, radio-therapy inevitably causes damage to the normal head and neck tissues including nerve structures (brain stem, spinal cord, and brachial plexus), mucous membranes, and swallowing muscles. Current commercially-available remedies as well as therapeutic interventions provide only temporary symptom relief, hence, do not address irreversible glandular damage. Further, despite salivary gland-sparing techniques and modified dosing strategies, long-term hypo-function remains a significant problem. Although a single governing mechanism of radiation-induced salivary gland tissue damage and dysfunction has not been yet elucidated, the potential for synergy in radio-protection (mainly, and possibly -reparation) via a combinatorial approach of mechanistically distinct strategies, has been suggested and explored over the years. This is, undoubtfully, in parallel to the ongoing efforts in improving the precision, safety, delivery, and efficacy of clinical radiotherapy protocols/outcomes, and in designing, developing, evaluating and optimizing (for translation) new artificial intelligence, technological and bio-pharmaceutical alternatives, topics covered in this review
Graphical abstract
Graphical abstract:
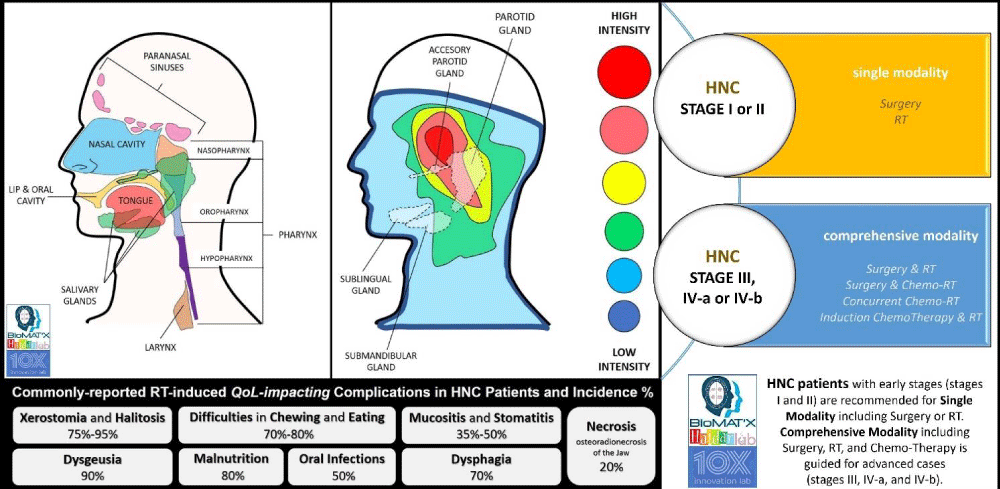
It is well recognized that the incidence of cancer, the second leading cause of death, globally, is increasing; an ongoing major burden of disease and public health burden, Worldwide. While there were 14.1 million cancer cases reported in 2012, the World Health Organization (WHO) estimated about 1 in 6 deaths is due to cancer, with 9.6 million such deaths reported in 2018. In the United States, today, cancer is the second leading, after heart disease, cause of death among men and women, with over 1 million new cases diagnosed, annually [1]. Despite a reduction in tobacco consumption and significant modern advancements in medicine, the number of new cancer cases, per year, is projected to rise to 22.2 million by 2030 [1]. Cancers, often squamous cell carcinomas/neoplasms, that involve the oral cavity, nostrils, paranasal sinuses, naso-/oro-/hypo-pharynx, larynx, and the salivary glands, are common/collectively (despite their heterogeneity) termed head and neck cancers (HNC), which, together are responsible for nearly 200,000 deaths, a year, Worldwide [2]. In the United States alone, HNC represents 4% - 5% of all cancers, and in Europe, HNC is the 6th most frequent group of cancers [3]. Besides the alarming incidence and mortality rates, HNC suffers a relatively poor prognosis, overall, whether due to delays in diagnosis, staging, treatment, particulars of the tumor site, onset, type of symptoms, and/or efficacy of therapies, to mention a few. Such factors further contribute to permitting the progress and upstaging of the malignant tumor(s) which eventually result in enfeebled survival, despite the application of novel or advanced intensive therapeutic regimens. Briefly, treatment, often a multi-disciplinary case-specific approach, can employ chemo-/radio-/immune-therapy, surgery, or combinatorial strategies [4]. Herein, radiotherapy (RT), whether radical or prophylactic, remains a mainstay of HNC treatment, especially in light of modern improvements in precisely targeting and delivering the required radiation doses to the tumor, thereby allowing additional sparing of normal/healthy surrounding tissue(s), greatly reducing side or adverse effects of radiation, and consequently improving the quality of life (QoL) of patients as well as their families [5-8]. IMRT (intensity‐modulated radiotherapy), VMAT (volumetric modulated arc therapy), and particle (ion-based) therapy are perhaps fine examples of modern high-precision RT [7]. Indeed, it has been estimated that > 80% of HNC patients exhibit xerostomia and salivary gland hypofunction following RT [3,4], in which, as well, depending on the RT dose, delivery method, and employed salivary gland-sparing techniques, 64% - 91% of irradiated HNC patients, often suffer from chronic xerostomia [3,4,8].
RT, in general, aims to realize localized destruction and control of the target tumor (-cells) and halt the reproductive potential, while minimizing toxicity onset. Specifically, high-energy radiation is deposited, causing DNA strands to break thereby damaging the cell genome either directly or indirectly (via free-radical production) and subsequently resulting in apoptosis, mitotic cell death, and tissue hypoxia, through different cascades and processes [5,7]. Depending on the radiation dose and tissue turnover, amongst other factors, RT can almost always be expected to result in a range of side effects, of which some are reversible, and others are irreversible (Figure 1). Indeed, HNC and oral squamous cell carcinoma (OSCC) patients receiving RT often experience pain, taste disturbances, difficulties in mastication and deglutition (swallowing) and suffer from mucositis, fungal infections, dental decay, alterations in speech, all of which are mainly due to or linked to salivary gland dysfunction which in turn results in hyposalivation and xerostomia [9-12]. Herein, xerostomia, a dry mouth sensation, is one of the main complications and complaints for HNC patients receiving RT, mainly as a sequela of the un-avoidable damage to the parotid and sub-mandibular glands (both produce over 80% of saliva) anatomically located with the radiation zone [8,12]. Inflammation, fibrosis, atrophy, and the reduced wound healing response, i.e. reparative and regenerative capacity of the glands, mainly due to lack of functional salivary gland stem/progenitor cells post-irradiation, render the inevitable radiotherapy-induced salivary gland damage and dysfunction, whether occurring early or late, a significant impediment to the QoL and survival of HNC and OSCC patients [10,13,14]. Hence, besides modern advancements in radiation engineering technologies, ample pharmacological and pharmaceutical solutions have been explored [14]. Accumulating knowledge in understanding the underlying signaling pathways, cellular and tissue responses, spatiotemporally, fuel the continuing efforts aimed to explore, develop and translate novel/innovative solutions to support the prevention (and treatment of) radiation‐induced side-effects and damage of salivary glands, the main focus of this review, designed to provide the clinical reader with a summary of relevant literature and recent innovative developments in salivary gland radioprotection and potential salivary gland repair, post-RT.
Figure 1: Head and Neck Cancers regions and irradiation intensity risk during HNC radiotherapy.
Saliva and salivary glands: pre-, during- and post-RT
Briefly, exocrine salivary glands are classified as either major (parotid, sub-mandibular, and sub-lingual) or minor (labial and buccal gland, glosso-palatine gland, and palatine and lingual) glands. Anatomically, all three major glands are highly vascularized, and innervated and are architecturally similar featuring a ductal structure with a secretory/excretory (saliva-producing acini surrounded by myoepithelial cells, myofibroblasts, immune cells, stromal cells, endothelial cells, and nerve fibers) opening into the oral cavity/mouth [15]. The glands differ in their type of acinar cells and as a result, in the type of produced saliva. While the parotid is composed of only serous acini thereby producing watery saliva, the sub-mandibular and sub-lingual glands contain a mix of serous and mucous (glycoprotein- rich) acini, thereby producing saliva of a different composition, a seromucous secretion. Secretion of saliva is stimulated by the sympathetic (proteins) and parasympathetic (serous/ions) branches of the autonomic nervous system [15,16]. Saliva is basically an oral lubricant fluid with multiple digestive functions critical for oro-dental health, QoL, and general well-being. It is composed of a complex mixture of water (99%), electrolytes (sodium, potassium, calcium, magnesium, etc ...), mucins, proteins, white blood cells, epithelial cells, immunoglobulins, anti-microbials/-bacterial and enzymes (1%) [16-18]. Hence, saliva is essential for moistening, chewing, swallowing, and chemically-digesting foods. It also facilitates speaking, aids the tongue in taste sensing, helps protect the oral mucosa (localized immunity/mucosal resistance), and plays a role in tissue re-mineralization. A healthy adult produces/secretes a daily average of 0.5 L - 1.5 L, at differential rates over the day, and at a near-neutral (buffer) pH of 6.7 [15-17,19-22]. Therefore, alterations in quantity (↓: hypo- or ↑: hyper-salivation) or quality of the secreted/produced saliva are associated with a variety of conditions and diseases and have been associated with some medications and therapies [23]. For instance, sialorrhea is a general term used for hyper-salivation (or drooling), often as a result of medication, systemic diseases, psychiatric disorders, and/or oral pathologies, amongst others [14]. It is also often linked to conditions such as Parkinson’s, epilepsy, amyotrophic lateral sclerosis or ALS, cerebral palsy, developmental disabilities, pregnancy, and/or drugs including clozapine [16,24] Common treatments for sialorrhea include surgical intervention, radiation of the salivary glands (to halt and diminish its function) and the use of oral anti-cholinergic drugs (to inhibit saliva production), however with known side or adverse effects. In recent years, numerous studies investigated the use of neuro-toxins, mainly botulinum neurotoxins or BoNTs, which basically are bacterial exotoxins that interfere with and block the exocytotic release of vesicular neuro-transmitters cholinergic neuromuscular activity in the target tissue, including commercially-available RimabotulinumtoxinB (RimaBoNT-B; FDA approval in 2000) and IncobotulinumtoxinA (IncoBoNT-A; FDA approval in 2010) in patients suffering sialorrhea, with attractively promising results [24,25]. On the other hand, salivary gland hypofunction (progressive loss of gland function) is commonly described or associated with the reduction of salivary flow and production, quantitatively. Frydrych [26] discussed salivary gland hypofunction etiology and classified causes into seven major areas; developmental, autoimmune/chronic inflammatory, endocrine, neurological/psychiatric, metabolic, infectious, and iatrogenic [26]. In a healthy individual, the un-stimulated “whole” salivary flow rate is averaged at 0.35 mL saliva per minute, with abnormalities indicated if the rate drops. For example, one of the most prevalent and studied diseases or disorders of the salivary gland is Sjögren’s syndrome (SS), a chronic auto-immune inflammatory reaction characterized by lymphocytic infiltration of the exocrine glands (mostly to the salivary or lacrimal glands), which generates a significant reduction in salivary flow rate - to below 0.1 mL whole saliva per minute secreted, un-stimulated [27]. It is perhaps noteworthy herein that the whole saliva indicates the collection of saliva (secreted from all salivary glands) present in the mouth. Other quantification techniques will require direct collection from the specific gland. Moreover, often it is reported in diagnosing SS that just the un-stimulated whole saliva flow rates are employed.
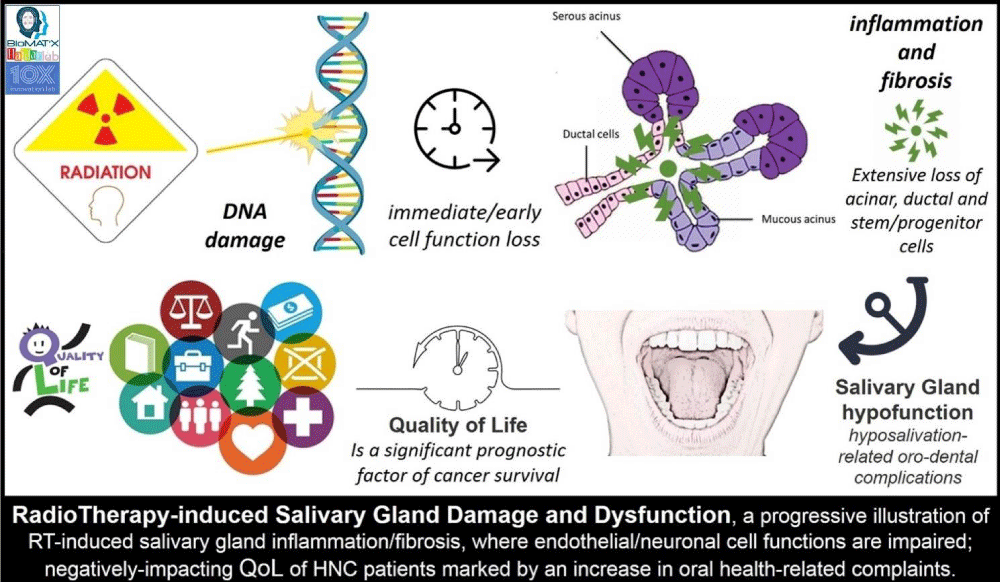
Hypo-salivation, therefore, is salivary flow rate reduction, quantified, clinically via sialometry. Xerostomia, on the other hand, is the reported perception or sensation, subjectively, of oral dryness. Hypo-salivation may or may not be accompanied by xerostomia and vice versa. Dryness in the mouth can be a side-effect of medications or due to diseases such as HIV/AIDS, diabetes, hypertension, and/or other factors including smoking, dehydration, mouth breathing, aging, and/or head and neck irradiation [14,16,23,28,29]. Indeed, xerostomia is one of the most commonly reported (and to be inevitably expected) complications of RT (during and after RT) for HNC, and as mentioned earlier, mainly as a predictable consequence to the significant damage (and generated inflammatory immune response) caused to the salivary glands which are located and included within the RT-zone or field [30-32]. RT, besides impairing salivary gland function and salivary flow rate, impacts the quality of the secreted saliva, given the loss or atrophy of acinar and ductal cells and granules (and stem/stromal and progenitor cells) and the consequential morphological changes to salivary fluid quality (including pH and buffering capacity), thereby affecting the essential protective, functional, and overall physiologic processes (Figure 2). Such damage [32] and impact can appear as soon as one week after the first RT session (acute RT-induced damage is due to a disturbance in the involved signal transduction pathways on the cell membrane). A progressive decrease in salivary gland function is evident with more RT sessions (delayed or late RT-induced damage is due to apoptosis-driven parenchymal cell loss, inflammation, blood vessel dilation, function loss, nerve injury, and reduced parasympathetic nervous function, and fibrosis) rendering rescue, repair, and regeneration rather challenging [33-35]. As a result, the QoL of a large proportion of patients receiving RT is severely compromised [36,37], with thicker or more viscous saliva and xerostomia leading to reported complaints [38]. Indeed, RT-related biochemical and proteomic alterations where several key glycoproteins, proteins, and other molecules are affected have been identified [31,39]. For example, Jehmlich, et al. [40], discussed such variations post-RT, detected significant alterations in 48 proteins, and highlighted the development of oral mucositis as a result of salivary gland dysfunction. The psycho-social and emotional impact on QoL of HNC patients, especially the elderly [41], where they experience and suffer from a compromised ability to taste, chew and swallow foods extended to their forced switching of dietary preferences to soft and carbohydrate-rich foods, thereby resulting in serious nutritional deficiencies [38,40,41]. Hyposalivation and sequential xerostomia also affect speaking and communication abilities, and patients experience nocturnal oral discomfort, consequently, causing additional stress and leading to withdrawal from the needed every day or day-to-day societal and emotional interactions [42-44]. Furthermore, with the prolonged oral clearance of sugars, the oral mucosa becomes painfully dry, sticky, and more susceptible to infection, the progression of dental caries (tooth decay), gingival and periodontal disease and trauma, accentuating the importance of oro-dental hygiene and care, especially in the elderly patients [41]. Other sequelae include erosion and ulceration of mucosal tissues, oral candidiasis, dysgeusia and dysphagia. Therefore, it is common for HNC patients to suffer from depression, feelings of anguish, and anxiety after receipt of the RT protocol [14,37,45-49]. While the recovery of irradiated salivary glands at the cellular and molecular has been thus far shown to be limited, salivary recovery post-RT, from our clinical exposure and expertise, is a possible, yet lengthy (> 3 years), dire and capricious process; with underlying mechanisms not yet fully understood.
Figure 2: Progression of RT-induced salivary gland damage and dysfunction in HNC patients.
Radiation-induced damage prevention and potential repair/regeneration of salivary glands
Understanding the underlying mechanisms governing cellular and molecular control of salivary gland function is highly pertinent, during- and post-RT, to aid in developing suitable and effective therapies, whether preventive or reparative. To date, it is safe to state that available therapies continue to be symptomatic, and no definitive solution or approach has been shown to compensate for and/or recover the impairment of salivary glands and function. Lifestyle modifications, synthetic saliva, and/or use of salivary stimulants and sialagogues, suffer shortcomings and are not satisfactory to our patients, as they either only provide temporary (short-term) relief or might have other disquieting side effects. Hence, global attention has been diverted to seeking and developing alternative novel methods, tools, and therapies, to offer to HNC patients undergoing RT that can provide superior long-term efficacy. Herein, tissue engineering, regenerative medicine, pharmaceutics, and nanotechnology may contribute.
Tissue engineering and reparative/regenerative medicine: current regimens and strategies
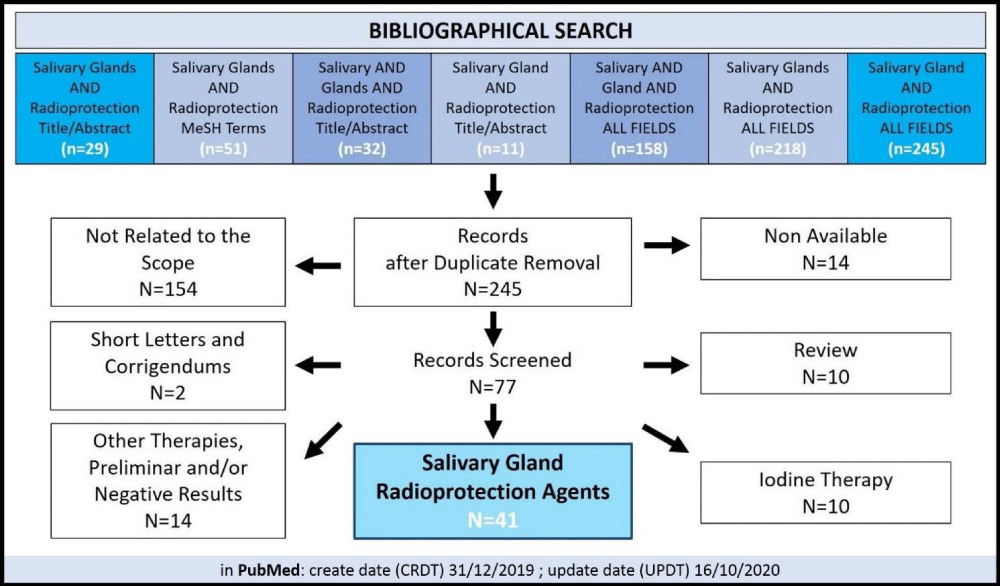
Several tissues and organs are highly sensitive to irradiation, such as the skin, esophagus, and bone marrow. However, the salivary glands are intricately radiosensitive, given their highly-differentiated cell content marked with a very low or slow proliferative rate [50]. This can help explain why the salivary glands, in specific, are somewhat unique in their early- and delayed effects post-RT, when compared to other tissues and organs. Nonetheless, salivary gland dysfunction and/or hypofunction have been shown, in some cases, to be reversible. Such treatment intervention is multi-factorial and highly dependent on original causality, for example, in cases of alcohol abuse and dehydration or hypothyroidism. RT-induced salivary gland damage and dysfunction is a far more challenging scenario. Auto-immune/chronic inflammatory diseases, such as SS or systemic lupus erythematosus also result in irreversible damage to the salivary glands [26]. Today, as mentioned earlier, only palliative and efficacy-limited regimens are commercially-available [47]. Tables 1-4 highlight a selection of various radio-protection strategies, at different stages of development, pre-clinically (in vitro and in in vivo testing) and clinical (human clinical trials). Briefly, a database search was performed in PubMed-indexed articles using a multi-search of the following keywords: “Salivary Glands AND Radioprotection [Title/Abstract]”, “Salivary Glands AND Radioprotection [MeSH]”, “Salivary AND Glands AND Radioprotection [Title/Abstract]”, “Salivary Gland AND Radioprotection [Title/Abstract]”, “Salivary AND Gland AND Radioprotection [ALL FIELDS]”, “Salivary Glands AND Radioprotection [ALL FIELDS] and “Salivary Gland AND Radioprotection [ALL FIELDS]”. Eligibility and inclusion criteria included English articles reporting radio-protection data from in vitro, in vivo, and/or clinical settings/trials. Articles dated back to 1978 up to the search end date of October 16th of 2020 were analyzed. Reviews, communications, or articles with preliminary results were not included in our analysis (Figure 3). Herein, our purpose is to screen the available literature and assess the level of development of new strategies, regimens and/or innovative solutions, to provide a usable prior-Art formatted report. Hence, not all included articles, which are tabulated for the reader, were aimed to be presented and dissected to be discussed in detail. This review attempts to provide an overview of the current understanding, status, and prospect of salivary gland radioprotection systems, with a look onto potential reparative and regenerative keys; where we, amongst other clinicians and researchers, do aspire for a superior, safe, efficacious, and long-term innovative solution that reverses RT-induced damage to the salivary glands of our HNC patients. Moreover, we opted to avoid concluding our overview with calls for additional research or validation; given that vital tissue engineering strategies employing the design, characterization, and optimization of novel biomaterials (and three-dimensional or 3-D printing), that can also be housing/incorporating release-controlled nanoparticles or nanocapsules that also are designed to encapsulate distinct mesenchymal stem cells, induced pluripotent stem cells (iPSCs), growth factors or cytokines and/or pharmaceutical agents or drugs, currently investigated at different levels of development are limitless in distinctions and details.
| Agent | Main Findings | Ref |
| bFGF-PLGA microspheres | Administration of basic Fibroblast Growth Factor (bFGF) prior to and immediately after irradiation, partially protected (44%) the rat parotid gland. | [69] |
| pH-responsive nanoparticles for active siRNAs delivery | The introduction of siRNAs specifically targeting the Pkcδ or Bax genes significantly blocked the induction of these pro-apoptotic proteins that normally occur post-irradiation in cultured salivary gland cells. The level of cell death from subsequent irradiation was significantly decreased. | [101] |
| rhHGF | Treatment of irradiated hPTS with recombinant human Hepatocyte Growth Factor (rhHGF) restored salivary marker expression and secretory function of hPTS. Changes in the phosphorylation levels of apoptosis-related proteins through the HGF-MET axis inhibited irradiation-induced apoptosis. | [102] |
| TIGAR over-expression | TIGAR (a p53-inducible regulator of glycolysis and apoptosis) over-expression could diminish the radio-sensitivity of Hs 917.T cells and decrease the autophagy level induced by ionizing irradiation. | [103] |
| Table 2: Radioprotection of salivary glands, in vivo using murine models. | ||
| MURINE | ||
| Agent | Main Findings | Ref |
| Keratinocyte Growth Factor-1 (KGF-1) | Local delivery of keratinocyte growth factor-1 into irradiated salivary glands protected RT-induced salivary cell damage, suppressed p53-mediated apoptosis, and prevented salivary hypofunction. | [72] |
| pH-responsive nanoparticles complexed with siRNAs | Knockdown of Pkcδ reduced the number of apoptotic cells during the acute phase of irradiation damage and markedly improved salivary secretion at 3 months. | [101] |
| Dasatinib / Imatinib | Delivery of dasatinib or imatinib resulted in >75% protection/rescue of salivary gland function at 60 days end-point. Continuous dosing with dasatinib extended protection to at least 5 months and was correlated with histologic evidence of regenerated salivary gland acinar cells. | [104] |
| Human Adipose tissue-derived Mesenchymal Stem Cells (AdMSCs) | Local transplantation of AdMSCs improved tissue remodeling following irradiation-induced damage in salivary gland tissue. The use of a carrier enhanced the effects of AdMSC-mediated cellular protection against irradiation via paracrine secretion. | [105] |
| Botulinum Toxins (BTX) | Irradiated mice showed a 50% reduction in salivary flow after 3 days, whereas mice pre-injected with BTX had a 25% reduction in salivary flow rate (ƿ<0.05). BTX pre-treatment ameliorates RT-induced salivary gland dysfunction. | [106] |
| AdMSCs secretome | Secretome modulated by hypoxic conditions to contain therapeutic factors contributed to salivary gland tissue re-modeling and demonstrated a potential to improve the consequences of RT-induced salivary hypofunction. | [107] |
| Resveratrol (RES) | Administration of RES reversed the reduction of saliva secretion induced by irradiation and restored salivary amylase and superoxide dismutase activity. RES can protect salivary glands against the negative effects of irradiation. | [108] |
| Amifostine | Amifostine alleviated the effects of irradiation on the bio-functions of cells, such as organelles, highly-involved in the secretory process. Amifostine can alleviate xerostomia caused by the late or delayed effects of irradiation. | [109] |
| Serotype 5 Adenoviral (Ad5) vector-mediated transfer of basic Fibroblast Growth Factor (AdbFGF) or Vascular Endothelial Growth Factor (AdVEGF) complementary DNAs | Single local administration of a modest dose (5 × 109 particles/gland) of a serotype 5 adenovirus (Ad5) vector encoding either bFGF or VEGF prior to irradiation, prevents rapid micro-vessel density loss in salivary glands and reduces the loss in salivary flow rate (as measured 8 weeks post-RT). | [110] |
| Tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl) | Tempol treatment was found to protect salivary glands significantly against radiation damage (approximately 60% improvement); with no tumor protection observed. | [111] |
| Tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl) | Tempol treatment pre-irradiation significantly reduced RT-induced salivary hypofunction (approximately 50-60%). Tempol (I.V. or S.C.) administration also showed significant radioprotection. Topical use of tempol, either as a mouthwash or gel, was also reported to be radioprotective. | [112] |
| Isoproterenol (IPR) | IPR stimulates adenylate cyclase/cyclic AMP (AC/cAMP) to increase the level of cAMP,25 and then increases cellular membrane ion permeability, ion active transport, and protein biosynthesis. These events, together with the release of heavy metals, appear to reduce irradiation injury. | [113] |
| Tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl) | Irradiation resulted in a dose-dependent reduction of salivary flow rate in this mouse model. | [114] |
| WR-2721 WR-3689 WR779 13 |
Tumors examined take up fewer WR-3689 than the other two protectors. In RIF-1 tumors, WR-3689 is taken up most avidly, but the three drugs tend to be equally protective. | [115] |
| WR-2721 | There is potential for protecting dose-limiting, late-responding normal tissue in the RT of human tumors with both neutrons and conventional radiotherapy. | [116] |
| WR-1065 | Localized delivery to salivary glands markedly improved radioprotection at the cellular level. Also, mitigated the adverse side-effects associated with systemic administration. | [117] |
| Hypoxia pre-conditioned human Adipose tissue-derived Mesenchymal Stem Cells (hAdMSCs-HPX) | Results suggest that hAdMSCs-HPX protect salivary glands from RT‐induced apoptosis and preserve acinar structure and functions via the activation of FGFR‐PI3K signaling by actions of hAdMSC‐ secreted factors, including FGF-10. | [118] |
| Entolimod | At days 8 and 15, entolimod treatment led to noticeable mitigation of damage in salivary gland tissue. Treatment 1 hr post-RT irradiation seems more effective than 30 min pre-RT. | [119] |
| Statins (Simvastatin) | Administration of Simvastatin could delay and reduce the extent of elevation/over-expression of TGF-β1, which in turn protects the submandibular glands from RT-induced injury. | [120] |
| RAT | ||
| Agent | Main Findings | Ref |
| Se, Zn and Mn + Lachesis muta venom (O-LM) | O-LM prevented permanent submandibular gland alterations demonstrating promising results in radioprotection and recovery from RT-induced injury. | [121] |
| Pilocarpine, Methacholine, Reserpine and Methacholine + Reserpine | Pre-treatment with pilocarpine or methacholine improved all measured glandular functions. Pre-treatment with a combination of reserpine and methacholine showed additive protective effects on submandibular gland function, signifying cooperation of muscarinic and alpha-adrenergic receptors. | [122] |
| Phenylephrine Isoproterenol Methacholine or Methacholine + Phenylephrine |
Pre-treatment with phenylephrine, isoproterenol and methacholine combined with phenylephrine resulted in less irradiation damage to parotid gland functions as indicated by quantified lag phase and flow rate. | [123] |
| WR-2721 | WR-2721 provided a significant degree of protection for all glandular functional parameters including gland weight. | [124] |
| cAMP | The demonstrated substantial protective effect of exogenously-administered cAMP on the parotid gland supports the previously-suggested radioprotection mechanism by the beta-adrenergic agonist isoproterenol, which is known to elevate endogenous intracellular cAMP. | [125] |
| WR-2721 Isoproterenol |
The aminothiol WR-2721 and beta-adrenergic agonist isoproterenol both conferred considerable radioprotection to the rat parotid gland. Isoproterenol acts on the beta-receptor, and its specific antagonist, propranolol, eliminated the protective effect of isoproterenol, thereby implicating the beta-receptor and cAMP in the radioprotection mechanism. | [126] |
| WR-2721 | While non-protected glands suffered a drastic reduction in the amount of acinar tissue, ducts and blood vessels exhibited only minor morphological changes. Herein, WR-2721 protected the glands with similar signs of damage yet to a much lesser degree, in comparison. | [127] |
| WR-2721 | WR-2721 protected against the acute phase of irradiation damage manifested during the first week post-RT. The drug also protected against chronic damage, appearing later. | [128] |
| Thymol | Thymol at a dose of 50 mg/Kg significantly impacted (positively) salivary gland dysfunction caused by ionizing irradiation. Short- and late-side effects of RT on the salivary glands were considered reduced by Thymol in those rats. | [129] |
| TLK1B | After a single fraction of 16 Gy, the decline in salivary function at 8 weeks was less pronounced in TLK1B-treated animals (40%) when compared to saline-treated controls (67%). | [130] |
| TLK1B associated with rAAV9 | AAV2/ 9-TLK1B groups showed no decline in salivary flow post-irradiation (121% increase) and salivary flow was not significantly different in irradiated and non-irradiated animals treated similarly with TLK1B. | [131] |
| Table 3: Radioprotection of salivary glands, in vivo using non-murine models. | ||
| RABBIT | ||
| Agent | Main Findings | Ref |
| Lidocaine HydroChloride | Pre-treatment with lidocaine improved irradiation tolerance of both, parotid and submandibular glands. Ultra-structure was largely preserved. | [132] |
| Lidocaine Amifostine Pilocarpin |
Only animals pre-treated with lidocaine or Amifostine (alone or combined with pilocarpin) showed a slight non-significant reduction in the salivary ejection fraction. Lidocaine and Amifostine could largely preserve the glandular ultra-structure. | [133] |
| mini-PIG | ||
| Agent | Main Findings | Ref |
| Orciprenaline Carbachol |
Acinar cells of both glands were significantly more numerous in the pre-treatment group. Also, cells seemed better preserved. Yet, such effects were more pronounced in the parotid gland (appearing almost normal) than in the submandibular gland. | [134] |
| Adenoviral vector encoding FGF2 (AdLTR2EF1a-FGF2) | A single pre-administration of a hybrid serotype 5 adenoviral vector encoding FGF2 (AdLTR2EF1a-FGF2) resulted in the protection of parotid microvascular endothelial cells from irradiation damage and significantly limited the decline of parotid salivary flow. | [135] |
| Table 4: Radioprotection of salivary glands, clinically in human subjects. | ||
| Agent | Main Findings | Ref |
| WR-2721 | Administration of WR-2721 prior to each dose of irradiation was feasible and without significant toxicity at 100 mg/m2. Salivary gland function improved over time after the completion of RT, particularly in the parotid gland. | [136] |
| Botulinum Toxin A (BTX-A) |
The SUVmean of the 225Ac-labelled PSMA radio-ligand in the injected parotid gland (right) showed a highly significant decrease of up to 60% when compared with the left side in the 63 years old patient with advanced metastatic castration-resistant prostate cancer (suffering from sialorrhea) receiving 80 units of BTX-A. | [137] |
| Amifostine | Amifostine reduces acute xerostomia and mucositis. | [138] |
| SMGT + IMRT | Surgical submandibular gland transfer (SMGT) was combined with intensity-modulated radiotherapy (IMRT) in a prospective phase II feasibility trial, in a single institution, including 40 HNC patients. At 12 months post-RT, the rate of absent or only mild xerostomia was 89%, and salivary flow rates were approximately 75% of pre-RT levels. Hence, patients reported decreased xerostomia and improved QoL. | [139] |
| Helical Tomotherapy (HT) |
HT is described as an innovative, more precise, and less toxic RT technique using a continuously rotating gantry to integrate 3D image guidance (a linear accelerator with computerized tomography) and deliver IMRT in a helical pattern. In 175 HNC patients, followed for up to 36 months, HT was used to deliver irradiation doses to bi-lateral parotid glands (PG-T), contra-lateral submandibular gland (cSMG), and accessory salivary glands in the oral cavity. Xerostomia was significantly decreased when the mean doses of PG-T, cSMG, and OC were kept below 29.12Gy, 29.29Gy, and 31.44Gy, respectively. | [140] |
Figure 3: PRISMA flow diagram for the bibliographic electronic search on PubMed Central.
Palliative care for RT-induced salivary gland dysfunction: Current and commercially-available palliative options for HNC patients undergoing RT include chewing gum (sugar-free), saliva substitutes, oral and topical lubricants, malic and ascorbic acid, saliva stimulants and sialogogues such as pilocarpine (Salagen, for example) and cevimeline (Evoxac, for example). As mentioned above, none have proven to restore normal QoL and patient satisfaction, mainly due to their limited efficacy and effectiveness [30,42]. On top, adverse side effects are common, and such options are often costly to patients, requiring multiple daily uses over long periods of time. In parallel, patients, especially the elderly, institutionalized and frail, need to go through education and training to acquire new eating and lifestyle habits; learn to prevent or avoid impaired swallowing and potential choking, and improve their oral and dental hygiene practices and tools to prevent (or halt the progression of) dental and oral mucosal diseases, infections, and tooth loss. Other palliative care options including acupuncture and electro-stimulation (enhancement of salivary reflexes) are currently undergoing investigation [30,51].
The only Food and Drug Administration/FDA–approved radio-protective and anti-xerostomia drug for clinical use (adjuvant setting) is Amifostine; an organic thiophosphate, cryoprotective agent and free radical scavenger administered subcutaneously or most often intravenously upon reconstitution with normal saline prior to or simultaneously with RT to then accumulate within the salivary glands, has been extensively studied since its development, initially under the nuclear warfare program [14,52]. Today, while it continues to benefit some patients, prophylactically, via minimizing the effects of xerostomia and taste loss, it is often associated with severe side effects including a rapid decrease in blood pressure (hypotension), nausea, and emesis or vomiting. A recent analysis of several clinical trials associated Amifostine with low-quality and mixed evidence in preventing dry mouth complaints in patients receiving RT to the head and neck region, in the short- to medium-terms (up to three months post-RT) and has questioned its potential in tumor cell protection; thereby further narrowing its clinical safety and efficacy window, especially in light of its high cost [52,53]. Essentially, its use in radiation-induced xerostomia has already been cautioned in the year 2008 by the American Society of Clinical Oncology [54] and so, its controversial and debatable safety and use in all cancer cases linger.
Preventive and interventional care for RT-induced salivary gland dysfunction: The main objective of any planned and/or prescribed preventive option should be the relief of symptoms and complications associated with hypo-salivation and xerostomia in HNC patients scheduled to receive RT, in order to prevent deterioration in their QoL thereby enhancing their battle with/against underlying cancer, its treatment and consequences thereof [55]. As discussed earlier, despite advancements in irradiation techniques and regimens including IMRT, only palliative, and prophylactic options are currently available (commercially), all of which do suffer substantial shortcomings [56,57]. One might even consider IMPT or intensity-modulated proton therapy, used to deliver a much-reduced irradiation dose and subsequently less toxic than IMRT, thereby alleviating much of the typical side effects of RT; however, IMPT is recognized to be more expensive and lacks accessibility or availability [43,56,57].
Contemporary alternative strategies and solutions
1. Surgical intervention: To prevent RT-induced hyposalivation, sub-mandibular gland preservation, and protection from irradiation via surgical relocation to the sub-mental space, thereby away or out of the irradiation zone, has been explored, with positive results. It is perhaps worth mentioning herein that the sub-mandibular salivary gland supplies up to 90% of the unstimulated saliva formation/secretion. However, such highly-invasive interventional procedures are peculiar and require exquisite surgical manipulation skills and settings. Further, surgical transfer of salivary glands is not indicated or possible for cancers of the oral cavity or patients undergoing (systemic) chemotherapy. In addition, for the gland to either retain or restore functionality, the connection of the gland to the main duct must be maintained or restored, respectively [58]; altogether rendering it a very limit-ed/-ing option. In terms of innovative approaches, Rao, et al. [59] recently described the use of a synthetic hydrogel (TraceIT; composed of water and iodinated cross-linked polyethylene glycol), injected via an 18-gauge needle, to serve as a minimally-invasive “spacer” (previously demonstrated in the treatment of prostate cancer), and displace or relocate the sub-mandibular gland in order to protect it from irradiation toxicity and be able to deliver a reduced irradiation dose; however, the experimental model used herein comprised of four refrigerated cadaveric specimens and no further in vivo or clinical studies evaluated the usability, malleability, safety, and efficacy, amongst other central factors or parameters, in clinical organ spacing.
2. Tissue engineering and regenerative medicine: Clearly, better approaches need to be explored and developed, driving the search elsewhere, into the multi-disciplinary areas of tissue engineering and regenerative medicine, in order to combine with and improve current options or to innovate and translate new alternative solutions, for wound healing. This is especially true, in light of accumulating knowledge and understanding of the underlying mechanisms governing radiation-induced salivary gland damage and dysfunction [47]. Indeed, from inducing DNA damage (via a. the generation of ROS/reactive oxygen species or b. the breakage of the DNA double-strand) to mutations to cell death (by apoptosis or necrosis, depending on cell type, injury, and cellular responses), to the loss of salivary progenitors, to the accruing evidence regarding the regenerative capacity (slow yet existent) of salivary glands following RT-induced injury, more evidently upon the administration of stimuli (exogenous delivery of stem cells and/or growth factors, for example), altogether re-emphasize the potential of such complex yet innovative approaches in finding a better clinical alternative solution. In a recent clinical study, Ho, et al. [60] evaluated the effects of a commercially-available slowly-dissolving adhering disc/tablet formulation (OraCoat XyliMelts) on the oro-dental health, enamel remineralization, bio-film formation, saliva presence, pH and buffering in 5 patients diagnosed with xerostomia (criteria: un-stimulated whole saliva flow rate below 0.2 mL per minute and a stimulated saliva flow rate of less than 0.5 mL in 5 minutes). They also assessed the patient self-reported comfort with the mint-flavored, xylitol-releasing tablets. Subjects were instructed to use the disc as often as needed for dry mouth symptom relief. In the end, a mean of 4+1 discs each day and 2 discs each night, were used. Overall, desirable effects of the product on symptomatic alleviation and management of xerostomia were reported. The authors reported effective local palliation, reduced dental sensitivity, improved salivary production and buffering capacity, reduced plaque formation, and alleviated xerostomia symptoms, without the need to use any systemic sialagogue medications throughout the 21 days of the study [60]. Yet, this is a pilot study, limited to involving a small number of participants.
3. Biomaterials and cell therapy: One of the fundamental roles for the maintenance of the body of any living organism is regeneration, which enables the repair and restoration of lost or damaged tissue [47,61]. Adult stem/stromal and progenitor cells have been identified in many tissues and are known to have a key role in the regeneration and repair, initiated or activated either by the excessive loss of differentiated cells (pool) or via (niche) environmental cues. In the presence of functional biomaterials such as the previously-described injectable hydrogel spacer [59] and a feasible agent-delivery tablet or disc [60], would loading, encapsulating, or incorporating putative salivary progenitor or stem/stromal cells, for example, a distinct type of stimuli, yield better results? Supplying salivary gland progenitor and stem/stromal cells, via a proper release-controlled dose-responsive carrier, might be able to re-establish the disrupted salivary stem/progenitor cell pool and niche, restore glandular tissue homeostasis, reverse hypo-salivation, and perhaps control xerostomia, a hypothesis we are currently examining in our laboratory, employing natural and synthetic polymers, liposomes, solid lipid nanoparticles, and core-shell nanocapsules, and further supplemented by other pharmaceutical agents. Modern medicine and biomedical research aim to control and enhance radioprotective as well as regenerative and reparative capabilities through the utilization of cells (cell lineages or primary cells); growing surface control using bio-scaffolds and/or manipulating growth factor/cytokine concentrations [47,62]; strategies designed to stimulate residual cells to regenerate acini and other parenchymal elements (ductal ligation) and infiltrate growth factor doses to boost salivary gland repair post-RT [63].
4. Growth factor and cytokine therapy: Somatomedin C is a hormone, similar to insulin in molecular structure, and actually is better known as IGF-1 or insulin-like growth factor 1 [64]. While a statement as “increased insulin-like growth factor signaling induces cell proliferation, survival and cancer progression” is true, it is traditional and partial, to a great extent. Today we understand that the issue is much more complex. For instance, IGF regulates cellular senescence which is known to halt the proliferation of aged and stressed cells and does play a key role in cancer development. Actually, there is accruing evidence that, over time, IGF not only regulates but also induces premature cellular senescence (tumor suppressor protein p53-dependant, in terms of acetylation, stabilization, and activation) [65]. Hence, despite the understandably-alarming, at first and for some, suggestion to exogenously administer/supply cytokines and growth factors to sites of cancer, recent years have indeed witnessed a note-worthy increase in the study of growth factors as cytoprotectants including their use as radioprotectors for salivary glands, and to reduce RT-induced symptoms, such as oral mucositis. To date, various growth factors have emerged as potential radioprotectors, including neurotrophic factors [66,67], epidermal growth factor (EGF), fibroblast growth factor (FGF) [68,69], keratinocyte growth factor (KGF) [70,71] and the afore-mentioned insulin-like growth factor-1 or IGF-1 [72-74]. Meyer, et al. [73], for example, investigated and determined the radioprotectant and therapeutic effect of IGF-1, in a murine model. They found that IGF-1 is mediated by the activation and maintenance of a histone deacetylase, specifically the Sirtuin 1 (SirT-1). Pre-treatment with IGF-1 enabled the repair of double-stranded breaks in the DNA of parotid salivary gland cells within the first hours post-irradiation, thereby allowing for optimal DNA repair (i.e. IGF-1 promotes DNA repair in irradiated parotid salivary glands via the maintenance and activation of SirT-1) to fulfill the cell cycle checkpoints. However, hours later and as early as 8h, RT-induced apoptotic cells were detected [73]. Such observations lead to further study of the signaling cross-talk between IGF-1 and SirT-1, thereby identifying several activators, stabilizers, and inhibitors, including the afore-mentioned inhibition of the p53-mediated apoptosis and the phosphoinositide 3-kinase (PI3K) – protein kinase B (Akt) pathway [65], in-depth study-worthy topics, beyond the scope of this concise review. To date, studies, collectively indicate that cytokines can be radioprotective, and anti-apoptotic and suggest/promote that the exogenous and localized (via a release-controlled delivery system, preferably directly injectable) utilization of growth factors do stimulate endogenous stem cell populations/niche and will eventually contribute to the desired and/or pursued clinical solution suitable for preventing RT-induced damage, diminishing salivary hypo-function, and restoring salivary gland function in RT- HNC cases.
5. Gene transfer therapy: The utilization of gene transfer, DNA transmission, and cell transduction to produce high levels of transgenic protein in order to correct cellular dysfunction and/or induce a new cellular function, post-RT, is a wide area of investigation and development. Baum, et al. [75], utilized an adenoviral technique to transfer the Aquaporin-1 (AQP1) gene into the sub-mandibular gland, reporting an increase in salivary flow when compared to control viruses into a rat or mini-pig models [75,76]. Yet, key shortcomings continue to exist for non-viral as well as viral vectors [61], rendering translation for routine clinical use difficult. Likewise, the therapeutic potential of genetic modification and application of small-interfering RNAs or siRNA for the purpose of target gene silencing are intensively investigated, progressing from pre-clinical testing in animal models to ongoing clinical trials for cancer, lung disease, and liver damage in human subjects. Thus far, highly limited in salivary gland tissues and accompanied by significant safety concerns [50]. For example, AQP-1 gene transfer into the salivary glands via adenoviral vectors to treat disorders such as SS, yielded strong immune responses, mainly due to the limited or low efficiency of intra-cellular siRNA delivery [77,78]. Herein, similar to growth factors, cell therapy, and pharmaceutical agent administration, the availability of a reproducible, scalable, safe and effective, release-controlled carrier/vehicle suitable for therapeutic siRNA delivery, directly into the salivary gland, ensuring sufficient residency/retention, is a challenge.
Wnt/β-catenin pathway: radio-protective role and bio-effect in RT-induced salivary gland damage
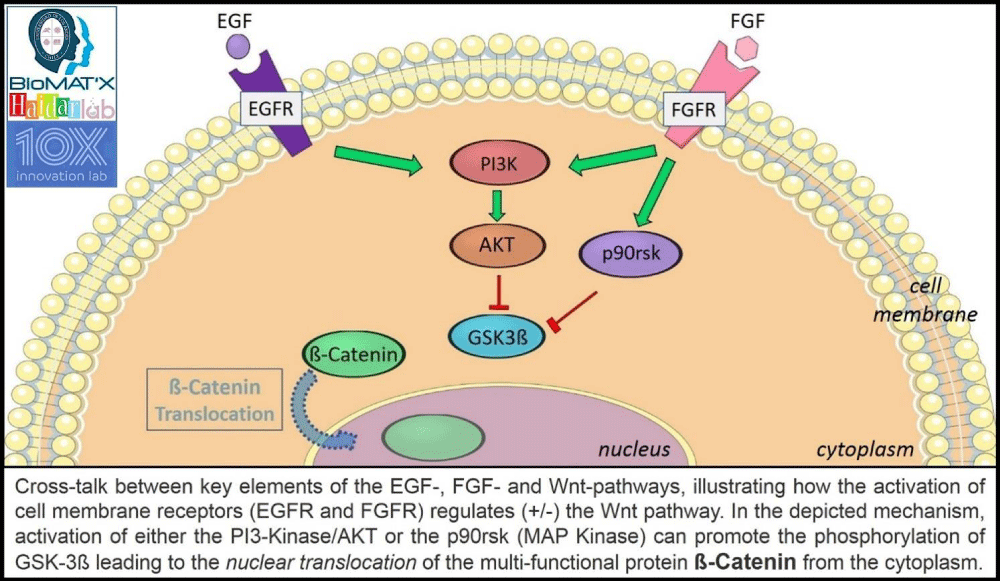
In existing irradiation studies and radio-protection literature, numerous cellular signaling pathways and cell-cycle alteration mechanisms have been explored. Of those, the Wnt/β-catenin signaling pathway seems to receive the utmost attention, recently, towards preventing the damage caused by irradiation [86]. Briefly, this canonical Wingless–Int (Wnt) pathway leads to the accumulation and translocation of co-activator β-catenin, a multi-functional protein involved in cell-cell adhesion, gene transcription, and physiologic homeostasis (adult), into the nucleus, via a series of molecular events initiated through the binding of specific Wnt proteins to the frizzled receptors on the cell surface. The pathway plays a critical role in cell regulating cell migration and determining cell fate, and mutations have been linked to human birth defects, cancer, and other disorders and diseases [79-83]. Activating the canonical Wnt/β-catenin signaling pathway is complex. It depends on a family of glycoproteins involved in cell-to-cell communication. To simplify, the interaction of ß-catenin with the cell adhesion molecule, e-cadherin, is involved in phenotypes: adhesion, mobility, and proliferation [80,81]. In absence of a Wnt ligand, β-catenin is degraded by the “destruction complex”. Several proteins are involved within this complex whereby Axin acts as a scaffold protein facilitating the interaction of Glycogen Synthase Kinase 3β (GSK-3β), Adenomatous Polyposis Coli (APC), and Casein Kinase 1α (CK1α), for β-catenin phosphorylation [82, 83]. Then, phosphorylated β-catenin is recognized by the β-transducin-repeat-containing protein (β-TrCP) and goes through the ubiquitin-proteasome degradation pathway. When the Wnt ligand activates Wnt signaling through the plasmatic membrane receptor frizzled with other lipoprotein receptors, the cytoplasmic protein disheveled (Dvl) is recruited and thereby activated. Herein, the activation of Dvl disrupts the “destruction complex” by dissociation of the GSK-3β from the Axin and inhibits the GSK-3β. As a result, β-catenin phosphorylation is also inhibited, allowing stabilization and translocation of β-catenin into the nucleus. Nuclear β-catenin then binds to a transcription factor-T cell factor and a lymphoid-enhancing factor (Tcf/Lef) and finally activates a response, i.e. changes in gene expression [79,84,85]. The Wnt signaling pathway cross-talks with other signaling pathways and can be modulated by several activators and inhibitors. For example, the utilization of growth factors, to activate or inhibit, has been extensively studied, further adding to the complexity given the wide range of involved genes [86]. Cross-talk between signaling pathways is possible via the common regulatory protein GSK-3β. For example, when the epidermal growth factor (EGF) is recognized by its native receptor (EGF-R), this complex activates the aforementioned phosphoinositide 3-kinase (PI3K) which facilitates the activation of AKT kinase regulator. Herein, the activation of AKT results in the inhibition of GSK-3β by phosphorylation [87-89] and ultimately leads to the translocation of β-catenin into the nucleus. On the other hand, the fibroblast growth factor (FGF) is also able to cross-talk with GSK-3β (common pathway with EGF) and the activation of its native receptor (FGF-R) is followed by PI3K which then results in the inhibition of GSK-3β via AKT activation [84,90]. Herein, FGF-R activation also involves MapK activation which inhibits GSK-3β through the p90 ribosomal protein s6 kinase (p90rsk) in an AKT-independent manner [91-93]. Therefore, activating the Wnt signaling pathway (Figure 4) through the utilization of cytoplasmic regulatory proteins (from other signaling pathways) is potentially able to promote β-catenin stabilization, its translocation to the nucleus, and the activation of survival genes [94]. Such understanding and revelations can lead to producing a plausible and innovative alternative strategy for the activation of native repair systems that may allow and promote the survival of the cells during and after RT. Possibly, can be even extended to explore the plausibility of prevention. To the best of my knowledge, Hakim, et al. [95] conducted one of the first/earliest clinical studies connecting signaling pathways (Wnt/β-catenin and TGF-β) with salivary gland irradiation damage. They reported an alteration in the expression pattern of Wnt1 in viable irradiated acinar cells of xerostomia patients, suggesting a possible therapeutic effect of the Wnt pathway in controlling RT-induced salivary gland damage and dysfunction [95], in accordance with previous in vitro studies [79]. Following this line of research, Hai, et al. [96] carried out a study analyzing the transient activation of the Wnt/β-catenin signaling pathway to prevent irradiation damage to the salivary glands. They reported, using a murine model, that activating the Wnt/β-catenin pathway through the transient activation of Wnt1 in the basal epithelium helped to prevent chronic salivary dysfunction generated by local irradiation, specifically via suppressing apoptosis and preserving or rescuing the life of salivary stem/progenitor cells. Salivation in experimental mice when compared to controls (animals receiving only RT) was increased/higher [79,96]. However, the radioprotective effect of Wnt/β-catenin activation seems, thus far, to only occur within a limited time lapse. Activating the signaling path 3 days before or 3 days after irradiation yielded dissimilar effects on the tissues [96]. Indeed, in another approach, the activation and modulation of a cell signaling pathway(s) using a cocktail (more than one) of activators has been suggested, with the Wnt signaling pathway (and its components) as a therapeutic target(s). Thula, et al. [69] evaluated the effect of EGF and bFGF (basic FGF) in salivary gland explants, reporting promising results regarding gland radioprotection [69]. Overall, taking the studied findings into account, it can be proposed that a Wnt/β-catenin signaling pathway activator might be a good candidate to be developed as a potential preventive and therapeutic strategy against RT-induced salivary gland damage in HNC patients. Herein, as was and is the present scenario with distinctive cells, proteins, cytokines, genes, growth factors, and drugs, a suitable/ideal delivery vehicle is once more, deemed vital.
Figure 4: EGF and FGF pathway(s) interaction with ß-catenin and canonical Wnt signaling pathway.
Closing expert remarks
Technology promise in translational tissue engineering and nanomedicine: The interplay between tissue engineering, regenerative medicine, biomaterials, bio-nanotechnology, and nanomedicine continues to be the hallmark of current scientific research Worldwide, promising to change every aspect of human life via creating revolutionary materials of biological origin for use in the diagnosis and treatment of devastating human diseases; a multi-disciplinary approach to innovative and translational solutions, suitable for scale-up, safe, efficacious and cost-effective routine clinical use [97-99]. Whether conventional small-molecule agents or emerging protein and/or peptide-based macromolecular biopharmaceutics, the therapeutic effect is of vital significance. Controlled or at least predictable delivery is also substantially necessary. An intense effort is invested into engineering such complex bio-systems capable to achieve optimum cell-material interactions while keeping intact the material’s bulk properties. One of the core interests of nanobiotechnology, for example, this decade has been drug/gene/cell bio-functional delivery, driving the design and development of bio-inspired, intelligent, or “smart” nano-systems [97,98,100]. It can be stated that a competitive and superiorly successful delivery system should offer: therapeutic outcome enhancement, patient compliance improvement, and overall cost reduction of therapy. For HNC cases suffering RT-induced salivary gland damage and dysfunction, an attractive delivery system, for clinical ease-of-use, can perhaps entail a directly injectable formulation, sterilizable, capable of efficiently holding a dose-responsive bio-load, maintaining its bio-activity over time, and “predictably” control its pharmaco-kinetic release profile.
Oxidative stress and ROS in RT-induced salivary gland damage and dysfunction: Although the mechanism of RT-induced salivary gland injury has not yet been determined, as previously mentioned throughout this review, recent studies have shown that RT-induced salivary gland damage and dysfunction are associated with the mechanisms of Aquaporin and activated water channel (AQP1 and AQP5) [141], calcium signaling (G-protein-coupled receptors, Ca2+ and Ca2+i) [142], micro-vascular injury (reduction in micro-vascular density or MVD: IR-induced DNA damage initiates ceramide generation via the activation of mitochondrial ceramide synthase and the de novo synthesis of ceramide) [143], cellular senescence (p16 INK4a/Rb-dependent pathway induced by growth/differentiation factor-15) [144] and apoptosis (phosphorylated PKCδ, caspase-3 and activating/inducing p53 expression and transcription) [145], which are closely related to oxidative stress. Indeed, RT significantly increases the levels of reactive oxygen species or ROS, thereby causing oxidative stress in the salivary glands; critical issues to consider for prevention and treatment strategies.
Artificial intelligence in HNC: For implementing a better diagnostic capability, standardizing reporting, quantifying scoring, and automating the work-flow as well as effective communication between surgeons, radiologists, pharmacists, and pathologists (146-148), for example, computational methods to detect patterns, make therapeutic (response to treatment) and prognostic predictions (i.e. machine learning or ML) using algorithms, big medical imaging datasets and digital image processing (including simple light microscopy) applying multi-factor analysis, conventional logistic regression and Cox analyses [146-149], have been increasingly advancing and attracting multi- and inter-/intra-disciplinary collaborations in recent years [146, 149]. Indeed, such can be combined with traditional strategies and approaches to further evolve and improve the accuracy or precision of detecting/diagnosing head and neck pre-cancerous and cancerous lesions [146,148,149]. Further, retrospective and radiomic (ML texture analysis of images) data would be a valuable tool to help predict prognosis or the course of HNC and provide treatment [146,147,149]. Today, the R&D&I trend (Figure 5) seems to focus more on exceeding the capabilities of human judgment [149] via designing novel data fusion ‘deep learning’ algorithms [148-150] that essentially would merge radiology, histology, and molecular measurements [149], from large-scale multi-centric prospective studies, for far more precise clinical predictions [150].
Figure 5: Artificial Intelligence, Machine and Deep Learning for potential RT in HNC patients.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding and acknowledgment
This SPECIAL ISSUE work was supported by operating grants provided to the HAiDAR R&D&I LAB/BioMAT’X (Laboratorio de Biomaterials, Farmacéuticos y Bioingeniería de Tejidos Cráneo Máxilo-Facial), member of CiiB (Centro de Investigación e Innovación Biomédica), Faculties of Medicine and Dentistry, Universidad de los Andes, Santiago de Chile, through the ANID-NAM (Agencia Nacional de Investigación y Desarrollo, Chile and National Academy of Medicine, USA) Grant código # NAM21I0022 (2020-2022), CORFO Crea y Valida I+D+i Grant código # 21CVC2-183649 (2021-2023), CORFO Crea y Valida — Proyecto de I+D+i Colaborativo - Reactívate” Grant código # 22CVC2-218196 (2022-2024), and FONDEF Concurso IDEA de I+D, ANID, Grant código # ID22I10215 (2022-2024). The author wishes to acknowledge the exceptional F-ODO students behind inspiring this piece: Yr3 (Andrea Bustos, Ismael Valenzuela, and Zabdiel Faundez), Yr4 (Alondra Beniscelli) and Yr6 (Ignacio Fernández). Finally, thanks to Ms. Lisa P., Publishing Manager-Journals at Heighten Science Publications Inc, USA, and colleagues for their incessant help and support.
- Cancer Fact Sheet World Health Organization. https://www.who.int/news-room/fact-sheets/detail/cancer. Accessed Oct 23, 2020.
- International Agency for Research on Cancer IARC. 2019, GLOBOCAN 2018, Cancer Incidence and Mortality Worldwide. Lyon: International Agency for Research on Cancer. http://gco.iarc.fr/today/fact-sheets-cancers. Accessed Aug 25, 2020.
- Yan K, Agrawal N, Gooi Z. Head and Neck Masses. Med Clin North Am. 2018 Nov;102(6):1013-1025. doi: 10.1016/j.mcna.2018.06.012. Epub 2018 Sep 20. PMID: 30342605.
- Cognetti DM, Weber RS, Lai SY. Head and neck cancer: an evolving treatment paradigm. Cancer. 2008 Oct 1;113(7 Suppl):1911-32. doi: 10.1002/cncr.23654. PMID: 18798532; PMCID: PMC2751600.
- Jaffray DA, Gospodarowicz MK. Radiation Therapy for Cancer. 2015. ISBN 9781464803499.
- Shetty AV, Wong DJ. Systemic Treatment for Squamous Cell Carcinoma of the Head and Neck. Otolaryngol Clin North Am. 2017 Aug;50(4):775-782. doi: 10.1016/j.otc.2017.03.013. PMID: 28755705.
- Barazzuol L, Coppes RP, van Luijk P. Prevention and treatment of radiotherapy-induced side effects. Mol Oncol. 2020 Jul;14(7):1538-1554. doi: 10.1002/1878-0261.12750. Epub 2020 Jun 24. PMID: 32521079; PMCID: PMC7332214.
- Gil Z, Fliss DM. Contemporary management of head and neck cancers. Isr Med Assoc J. 2009 May;11(5):296-300. PMID: 19637508.
- Deloch L, Derer A, Hartmann J, Frey B, Fietkau R, Gaipl US. Modern Radiotherapy Concepts and the Impact of Radiation on Immune Activation. Front Oncol. 2016 Jun 20;6:141. doi: 10.3389/fonc.2016.00141. PMID: 27379203; PMCID: PMC4913083.
- Baskar R, Dai J, Wenlong N, Yeo R, Yeoh KW. Biological response of cancer cells to radiation treatment. Front Mol Biosci. 2014 Nov 17;1:24. doi: 10.3389/fmolb.2014.00024. PMID: 25988165; PMCID: PMC4429645.
- Manukian G, Bar-Ad V, Lu B, Argiris A, Johnson JM. Combining Radiation and Immune Checkpoint Blockade in the Treatment of Head and Neck Squamous Cell Carcinoma. Front Oncol. 2019 Mar 6;9:122. doi: 10.3389/fonc.2019.00122. PMID: 30895168; PMCID: PMC6414812.
- Jensen SB, Vissink A, Limesand KH, Reyland ME. Salivary Gland Hypofunction and Xerostomia in Head and Neck Radiation Patients. J Natl Cancer Inst Monogr. 2019 Aug 1;2019(53):lgz016. doi: 10.1093/jncimonographs/lgz016. PMID: 31425600.
- Wu VWC, Leung KY. A Review on the Assessment of Radiation Induced Salivary Gland Damage After Radiotherapy. Front Oncol. 2019 Oct 17;9:1090. doi: 10.3389/fonc.2019.01090. PMID: 31750235; PMCID: PMC6843028.
- Miranda-Rius J, Brunet-Llobet L, Lahor-Soler E, Farré M. Salivary Secretory Disorders, Inducing Drugs, and Clinical Management. Int J Med Sci. 2015 Sep 22;12(10):811-24. doi: 10.7150/ijms.12912. PMID: 26516310; PMCID: PMC4615242.
- Ghannam MG, Singh P, Anatomy H , Salivary Glands N. StatPearls 2019 Available from https://www.ncbi.nlm.nih.gov/books/NBK538325/. Accessed Jan 29, 2020.
- Punj A. Secretions of Human Salivary Gland. Secretions of Human Salivary Gland, Salivary Glands - New Approaches in Diagnostics and Treatment, Işıl Adadan Güvenç, IntechOpen. 2018. doi: 10.5772/intechopen.75538. Available from: https://www.intechopen.com/books/salivary-glands-new-approaches-in-diagnostics-and-treatment/secretions-of-human-salivary-gland.
- Benn AM, Thomson WM. Saliva: an overview. N Z Dent J. 2014 Sep;110(3):92-6. PMID: 25265747.
- Tiwari M. Science behind human saliva. J Nat Sci Biol Med. 2011 Jan;2(1):53-8. doi: 10.4103/0976-9668.82322. PMID: 22470235; PMCID: PMC3312700.
- Proctor GB. The physiology of salivary secretion. Periodontol 2000. 2016 Feb;70(1):11-25. doi: 10.1111/prd.12116. PMID: 26662479.
- Qin R, Steel A, Fazel N. Oral mucosa biology and salivary biomarkers. Clin Dermatol. 2017 Sep-Oct;35(5):477-483. doi: 10.1016/j.clindermatol.2017.06.005. Epub 2017 Jun 27. PMID: 28916029.
- Farnaud SJ, Kosti O, Getting SJ, Renshaw D. Saliva: physiology and diagnostic potential in health and disease. ScientificWorldJournal. 2010 Mar 16;10:434-56. doi: 10.1100/tsw.2010.38. PMID: 20305986; PMCID: PMC5763701.
- Fábián TK, Beck A, Fejérdy P, Hermann P, Fábián G. Molecular mechanisms of taste recognition: considerations about the role of saliva. Int J Mol Sci. 2015 Mar 13;16(3):5945-74. doi: 10.3390/ijms16035945. PMID: 25782158; PMCID: PMC4394514.
- von Bültzingslöwen I, Sollecito TP, Fox PC, Daniels T, Jonsson R, Lockhart PB, Wray D, Brennan MT, Carrozzo M, Gandera B, Fujibayashi T, Navazesh M, Rhodus NL, Schiødt M. Salivary dysfunction associated with systemic diseases: systematic review and clinical management recommendations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007 Mar;103 Suppl:S57.e1-15. doi: 10.1016/j.tripleo.2006.11.010. PMID: 17379156.
- Dashtipour K, Bhidayasiri R, Chen JJ, Jabbari B, Lew M, Torres-Russotto D. RimabotulinumtoxinB in sialorrhea: systematic review of clinical trials. J Clin Mov Disord. 2017 Jun 6;4:9. doi: 10.1186/s40734-017-0055-1. PMID: 28593050; PMCID: PMC5460542.
- Jost WH, Friedman A, Michel O, Oehlwein C, Slawek J, Bogucki A, Ochudlo S, Banach M, Pagan F, Flatau-Baqué B, Dorsch U, Csikós J, Blitzer A. Long-term incobotulinumtoxinA treatment for chronic sialorrhea: Efficacy and safety over 64 weeks. Parkinsonism Relat Disord. 2020 Jan;70:23-30. doi: 10.1016/j.parkreldis.2019.11.024. Epub 2019 Nov 26. PMID: 31794936.
- Frydrych AM. Dry mouth: Xerostomia and salivary gland hypofunction. Aust Fam Physician. 2016 Jul;45(7):488-92. PMID: 27610431.
- Azuma N, Katada Y, Kitano S, Sekiguchi M, Kitano M, Nishioka A, Hashimoto N, Matsui K, Iwasaki T, Sano H. Correlation between salivary epidermal growth factor levels and refractory intraoral manifestations in patients with Sjögren's syndrome. Mod Rheumatol. 2014 Jul;24(4):626-32. doi: 10.3109/14397595.2013.850766. Epub 2013 Nov 5. PMID: 24252043.
- Millsop JW, Wang EA, Fazel N. Etiology, evaluation, and management of xerostomia. Clin Dermatol. 2017 Sep-Oct;35(5):468-476. doi: 10.1016/j.clindermatol.2017.06.010. Epub 2017 Jun 27. PMID: 28916028.
- Tan ECK, Lexomboon D, Sandborgh-Englund G, Haasum Y, Johnell K. Medications That Cause Dry Mouth As an Adverse Effect in Older People: A Systematic Review and Metaanalysis. J Am Geriatr Soc. 2018 Jan;66(1):76-84. doi: 10.1111/jgs.15151. Epub 2017 Oct 26. PMID: 29071719.
- Vissink A, Mitchell JB, Baum BJ, Limesand KH, Jensen SB, Fox PC, Elting LS, Langendijk JA, Coppes RP, Reyland ME. Clinical management of salivary gland hypofunction and xerostomia in head-and-neck cancer patients: successes and barriers. Int J Radiat Oncol Biol Phys. 2010 Nov 15;78(4):983-91. doi: 10.1016/j.ijrobp.2010.06.052. PMID: 20970030; PMCID: PMC2964345.
- Schaue D, Kachikwu EL, McBride WH. Cytokines in radiobiological responses: a review. Radiat Res. 2012 Dec;178(6):505-23. doi: 10.1667/RR3031.1. Epub 2012 Oct 29. PMID: 23106210; PMCID: PMC3723384.
- Williams JP, McBride WH. After the bomb drops: a new look at radiation-induced multiple organ dysfunction syndrome (MODS). Int J Radiat Biol. 2011 Aug;87(8):851-68. doi: 10.3109/09553002.2011.560996. Epub 2011 Mar 21. PMID: 21417595; PMCID: PMC3314299.
- Mohammadi N, Seyyednejhad F, Alizadeh Oskoee P, Savadi Oskoee S, Mofidi N. Evaluation of Radiation-induced Xerostomia in Patients with Nasopharyngeal Carcinomas. J Dent Res Dent Clin Dent Prospects. 2007 Summer;1(2):65-70. doi: 10.5681/joddd.2007.011. Epub 2007 Sep 10. PMID: 23277836; PMCID: PMC3525927.
- Strojan P, Hutcheson KA, Eisbruch A, Beitler JJ, Langendijk JA, Lee AWM, Corry J, Mendenhall WM, Smee R, Rinaldo A, Ferlito A. Treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat Rev. 2017 Sep;59:79-92. doi: 10.1016/j.ctrv.2017.07.003. Epub 2017 Jul 18. PMID: 28759822; PMCID: PMC5902026.
- Franzén L, Funegård U, Ericson T, Henriksson R. Parotid gland function during and following radiotherapy of malignancies in the head and neck. A consecutive study of salivary flow and patient discomfort. Eur J Cancer. 1992;28(2-3):457-62. doi: 10.1016/s0959-8049(05)80076-0. PMID: 1591063.
- Siddiqui F, Movsas B. Management of Radiation Toxicity in Head and Neck Cancers. Semin Radiat Oncol. 2017 Oct;27(4):340-349. doi: 10.1016/j.semradonc.2017.04.008. PMID: 28865517.
- Berk LB, Shivnani AT, Small W Jr. Pathophysiology and management of radiation-induced xerostomia. J Support Oncol. 2005 May-Jun;3(3):191-200. PMID: 15915820.
- Hammerlid E, Silander E, Hörnestam L, Sullivan M. Health-related quality of life three years after diagnosis of head and neck cancer--a longitudinal study. Head Neck. 2001 Feb;23(2):113-25. doi: 10.1002/1097-0347(200102)23:2<113::aid-hed1006>3.0.co;2-w. PMID: 11303628.
- Hall SC, Hassis ME, Williams KE, Albertolle ME, Prakobphol A, Dykstra AB, Laurance M, Ona K, Niles RK, Prasad N, Gormley M, Shiboski C, Criswell LA, Witkowska HE, Fisher SJ. Alterations in the Salivary Proteome and N-Glycome of Sjögren's Syndrome Patients. J Proteome Res. 2017 Apr 7;16(4):1693-1705. doi: 10.1021/acs.jproteome.6b01051. Epub 2017 Mar 24. PMID: 28282148; PMCID: PMC9668345.
- Jehmlich N, Stegmaier P, Golatowski C, Salazar MG, Rischke C, Henke M, Völker U. Differences in the whole saliva baseline proteome profile associated with development of oral mucositis in head and neck cancer patients undergoing radiotherapy. J Proteomics. 2015 Jul 1;125:98-103. doi: 10.1016/j.jprot.2015.04.030. Epub 2015 May 19. PMID: 25997676.
- Thomson WM. Dry mouth and older people. Aust Dent J. 2015 Mar;60 Suppl 1:54-63. doi: 10.1111/adj.12284. PMID: 25762042.
- Cereda E, Cappello S, Colombo S, Klersy C, Imarisio I, Turri A, Caraccia M, Borioli V, Monaco T, Benazzo M, Pedrazzoli P, Corbella F, Caccialanza R. Nutritional counseling with or without systematic use of oral nutritional supplements in head and neck cancer patients undergoing radiotherapy. Radiother Oncol. 2018 Jan;126(1):81-88. doi: 10.1016/j.radonc.2017.10.015. Epub 2017 Oct 27. PMID: 29111172.
- Li Y, Taylor JM, Ten Haken RK, Eisbruch A. The impact of dose on parotid salivary recovery in head and neck cancer patients treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2007 Mar 1;67(3):660-9. doi: 10.1016/j.ijrobp.2006.09.021. Epub 2006 Dec 4. PMID: 17141973; PMCID: PMC2001308.
- Jiang N, Zhao Y, Jansson H, Chen X, Mårtensson J. Experiences of xerostomia after radiotherapy in patients with head and neck cancer: A qualitative study. J Clin Nurs. 2018 Jan;27(1-2):e100-e108. doi: 10.1111/jocn.13879. Epub 2017 Jul 5. PMID: 28514511.
- Wang W, Xiong W, Wan J, Sun X, Xu H, Yang X. The decrease of PAMAM dendrimer-induced cytotoxicity by PEGylation via attenuation of oxidative stress. Nanotechnology. 2009 Mar 11;20(10):105103. doi: 10.1088/0957-4484/20/10/105103. Epub 2009 Feb 16. PMID: 19417510.
- Nadig SD, Ashwathappa DT, Manjunath M, Krishna S, Annaji AG, Shivaprakash PK. A relationship between salivary flow rates and Candida counts in patients with xerostomia. J Oral Maxillofac Pathol. 2017 May-Aug;21(2):316. doi: 10.4103/jomfp.JOMFP_231_16. PMID: 28932047; PMCID: PMC5596688.
- Kagami H, Wang S, Hai B. Restoring the function of salivary glands. Oral Dis. 2008 Jan;14(1):15-24. doi: 10.1111/j.1601-0825.2006.01339.x. PMID: 18173444.
- Villa A, Abati S. Risk factors and symptoms associated with xerostomia: a cross-sectional study. Aust Dent J. 2011 Sep;56(3):290-5. doi: 10.1111/j.1834-7819.2011.01347.x. PMID: 21884145.
- Bressan V, Bagnasco A, Aleo G, Catania G, Zanini MP, Timmins F, Sasso L. The life experience of nutrition impact symptoms during treatment for head and neck cancer patients: a systematic review and meta-synthesis. Support Care Cancer. 2017 May;25(5):1699-1712. doi: 10.1007/s00520-017-3618-7. Epub 2017 Feb 15. PMID: 28204992.
- Grundmann O, Mitchell GC, Limesand KH. Sensitivity of salivary glands to radiation: from animal models to therapies. J Dent Res. 2009 Oct;88(10):894-903. doi: 10.1177/0022034509343143. PMID: 19783796; PMCID: PMC2882712.
- de Castro G Jr, Guindalini RS. Supportive care in head and neck oncology. Curr Opin Oncol. 2010 May;22(3):221-5. doi: 10.1097/CCO.0b013e32833818ff. PMID: 20186057.
- Gu J, Zhu S, Li X, Wu H, Li Y, Hua F. Effect of amifostine in head and neck cancer patients treated with radiotherapy: a systematic review and meta-analysis based on randomized controlled trials. PLoS One. 2014 May 2;9(5):e95968. doi: 10.1371/journal.pone.0095968. PMID: 24788761; PMCID: PMC4008569.
- Riley P, Glenny AM, Hua F, Worthington HV. Pharmacological interventions for preventing dry mouth and salivary gland dysfunction following radiotherapy. Cochrane Database Syst Rev. 2017 Jul 31;7(7):CD012744. doi: 10.1002/14651858.CD012744. PMID: 28759701; PMCID: PMC6483146.
- Hensley ML, Hagerty KL, Kewalramani T, Green DM, Meropol NJ, Wasserman TH, Cohen GI, Emami B, Gradishar WJ, Mitchell RB, Thigpen JT, Trotti A 3rd, von Hoff D, Schuchter LM. American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J Clin Oncol. 2009 Jan 1;27(1):127-45. doi: 10.1200/JCO.2008.17.2627. Epub 2008 Nov 17. PMID: 19018081.
- Vissink A, Jansma J, Spijkervet FK, Burlage FR, Coppes RP. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14(3):199-212. doi: 10.1177/154411130301400305. PMID: 12799323.
- Braam PM, Terhaard CH, Roesink JM, Raaijmakers CP. Intensity-modulated radiotherapy significantly reduces xerostomia compared with conventional radiotherapy. Int J Radiat Oncol Biol Phys. 2006 Nov 15;66(4):975-80. doi: 10.1016/j.ijrobp.2006.06.045. Epub 2006 Sep 11. PMID: 16965864.
- Teng F, Fan W, Luo Y, Ju Z, Gong H, Ge R, Tong F, Zhang X, Ma L. Reducing Xerostomia by Comprehensive Protection of Salivary Glands in Intensity-Modulated Radiation Therapy with Helical Tomotherapy Technique for Head-and-Neck Cancer Patients: A Prospective Observational Study. Biomed Res Int. 2019 Jul 14;2019:2401743. doi: 10.1155/2019/2401743. PMID: 31380414; PMCID: PMC6662416.
- Marzouki HZ, Elkhalidy Y, Jha N, Scrimger R, Debenham BJ, Harris JR, O'Connell DA, Seikaly H. Modification of the submandibular gland transfer procedure. Laryngoscope. 2016 Nov;126(11):2492-2496. doi: 10.1002/lary.26029. Epub 2016 May 12. PMID: 27171786.
- Rao AD, Coquia S, De Jong R, Gourin C, Page B, Latronico D, Dah S, Su L, Clarke S, Schultz J, Rosati LM, Fakhry C, Wong J, DeWeese TL, Quon H, Ding K, Kiess A. Effects of biodegradable hydrogel spacer injection on contralateral submandibular gland sparing in radiotherapy for head and neck cancers. Radiother Oncol. 2018 Jan;126(1):96-99. doi: 10.1016/j.radonc.2017.09.017. Epub 2017 Oct 3. PMID: 28985953.
- Ho J, Firmalino MV, Anbarani AG, Takesh T, Epstein J, Wilder-Smith P. Effects of A Novel Disc Formulation on Dry Mouth Symptoms and Enamel Remineralization in Patients With Hyposalivation: An In Vivo Study. Dentistry (Sunnyvale). 2017 Feb;7(2):411. doi: 10.4172/2161-1122.1000411. Epub 2017 Feb 13. PMID: 28713645; PMCID: PMC5505684.
- Ogawa M, Oshima M, Imamura A, Sekine Y, Ishida K, Yamashita K, Nakajima K, Hirayama M, Tachikawa T, Tsuji T. Functional salivary gland regeneration by transplantation of a bioengineered organ germ. Nat Commun. 2013;4:2498. doi: 10.1038/ncomms3498. PMID: 24084982; PMCID: PMC3806330.
- Zhang NN, Huang GL, Han QB, Hu X, Yi J, Yao L, He Y. Functional regeneration of irradiated salivary glands with human amniotic epithelial cells transplantation. Int J Clin Exp Pathol. 2013 Sep 15;6(10):2039-47. PMID: 24133581; PMCID: PMC3796225.
- Okazaki Y, Kagami H, Hattori T, Hishida S, Shigetomi T, Ueda M. Acceleration of rat salivary gland tissue repair by basic fibroblast growth factor. Arch Oral Biol. 2000 Oct;45(10):911-9. doi: 10.1016/s0003-9969(00)00035-2. PMID: 10973565.
- Michalopoulou F, Petraki C, Philippou A, Analitis A, Msaouel P, Koutsilieris M. Expression of IGF-IEc Isoform in Renal Cell Carcinoma Tissues. Anticancer Res. 2020 Nov;40(11):6213-6219. doi: 10.21873/anticanres.14641. PMID: 33109558.
- Tran D, Bergholz J, Zhang H, He H, Wang Y, Zhang Y, Li Q, Kirkland JL, Xiao ZX. Insulin-like growth factor-1 regulates the SIRT1-p53 pathway in cellular senescence. Aging Cell. 2014 Aug;13(4):669-78. doi: 10.1111/acel.12219. Epub 2014 Apr 30. PMID: 25070626; PMCID: PMC4118446.
- Xiao N, Lin Y, Cao H, Sirjani D, Giaccia AJ, Koong AC, Kong CS, Diehn M, Le QT. Neurotrophic factor GDNF promotes survival of salivary stem cells. J Clin Invest. 2014 Aug;124(8):3364-77. doi: 10.1172/JCI74096. Epub 2014 Jul 18. PMID: 25036711; PMCID: PMC4109543.
- Swick A, Kimple RJ. Wetting the whistle: neurotropic factor improves salivary function. J Clin Invest. 2014 Aug;124(8):3282-4. doi: 10.1172/JCI77194. Epub 2014 Jul 18. PMID: 25036702; PMCID: PMC4109536.
- Kojima T, Kanemaru S, Hirano S, Tateya I, Suehiro A, Kitani Y, Kishimoto Y, Ohno S, Nakamura T, Ito J. The protective efficacy of basic fibroblast growth factor in radiation-induced salivary gland dysfunction in mice. Laryngoscope. 2011 Sep;121(9):1870-5. doi: 10.1002/lary.21873. Epub 2011 Aug 16. PMID: 22024837.
- Thula TT, Schultz G, Tran-Son-Tay R, Batich C. Effects of EGF and bFGF on irradiated parotid glands. Ann Biomed Eng. 2005 May;33(5):685-95. doi: 10.1007/s10956-005-1853-z. PMID: 15981868.
- Borges L, Rex KL, Chen JN, Wei P, Kaufman S, Scully S, Pretorius JK, Farrell CL. A protective role for keratinocyte growth factor in a murine model of chemotherapy and radiotherapy-induced mucositis. Int J Radiat Oncol Biol Phys. 2006 Sep 1;66(1):254-62. doi: 10.1016/j.ijrobp.2006.05.025. PMID: 16904525.
- Lombaert IM, Brunsting JF, Wierenga PK, Kampinga HH, de Haan G, Coppes RP. Keratinocyte growth factor prevents radiation damage to salivary glands by expansion of the stem/progenitor pool. Stem Cells. 2008 Oct;26(10):2595-601. doi: 10.1634/stemcells.2007-1034. Epub 2008 Jul 31. PMID: 18669914.
- Choi JS, Shin HS, An HY, Kim YM, Lim JY. Radioprotective effects of Keratinocyte Growth Factor-1 against irradiation-induced salivary gland hypofunction. Oncotarget. 2017 Feb 21;8(8):13496-13508. doi: 10.18632/oncotarget.14583. PMID: 28086221; PMCID: PMC5355115.
- Meyer S, Chibly AM, Burd R, Limesand KH. Insulin-Like Growth Factor-1-Mediated DNA Repair in Irradiated Salivary Glands Is Sirtuin-1 Dependent. J Dent Res. 2017 Feb;96(2):225-232. doi: 10.1177/0022034516677529. Epub 2016 Nov 16. PMID: 28106504; PMCID: PMC5331616.
- Grundmann O, Fillinger JL, Victory KR, Burd R, Limesand KH. Restoration of radiation therapy-induced salivary gland dysfunction in mice by post therapy IGF-1 administration. BMC Cancer. 2010 Aug 10;10:417. doi: 10.1186/1471-2407-10-417. PMID: 20698985; PMCID: PMC3087323.
- Baum BJ, Zheng C, Cotrim AP, Goldsmith CM, Atkinson JC, Brahim JS, Chiorini JA, Voutetakis A, Leakan RA, Van Waes C, Mitchell JB, Delporte C, Wang S, Kaminsky SM, Illei GG. Transfer of the AQP1 cDNA for the correction of radiation-induced salivary hypofunction. Biochim Biophys Acta. 2006 Aug;1758(8):1071-7. doi: 10.1016/j.bbamem.2005.11.006. Epub 2005 Dec 5. PMID: 16368071.
- Redman RS. On approaches to the functional restoration of salivary glands damaged by radiation therapy for head and neck cancer, with a review of related aspects of salivary gland morphology and development. Biotech Histochem. 2008 Jun;83(3-4):103-30. doi: 10.1080/10520290802374683. PMID: 18828044; PMCID: PMC2740375.
- Cotrim AP, Sowers A, Mitchell JB, Baum BJ. Prevention of irradiation-induced salivary hypofunction by microvessel protection in mouse salivary glands. Mol Ther. 2007 Dec;15(12):2101-6. doi: 10.1038/sj.mt.6300296. Epub 2007 Aug 28. PMID: 17726456.
- Guo L, Gao R, Xu J, Jin L, Cotrim AP, Yan X, Zheng C, Goldsmith CM, Shan Z, Hai B, Zhou J, Zhang C, Baum BJ, Wang S. AdLTR2EF1α-FGF2-mediated prevention of fractionated irradiation-induced salivary hypofunction in swine. Gene Ther. 2014 Oct;21(10):866-73. doi: 10.1038/gt.2014.63. Epub 2014 Jul 17. PMID: 25030610.
- Wang JF, Liu C, Zhang Q, Huang GH. Research progress in the radioprotective effect of the canonical Wnt pathway. Cancer Biol Med. 2013 Jun;10(2):61-71. doi: 10.7497/j.issn.2095-3941.2013.02.001. PMID: 23882420; PMCID: PMC3719192.
- Vidya Priyadarsini R, Senthil Murugan R, Nagini S. Aberrant activation of Wnt/β-catenin signaling pathway contributes to the sequential progression of DMBA-induced HBP carcinomas. Oral Oncol. 2012 Jan;48(1):33-9. doi: 10.1016/j.oraloncology.2011.08.008. Epub 2011 Sep 15. PMID: 21924667.
- Huang J, Qu Q, Guo Y, Xiang Y, Feng D. Tankyrases/β-catenin Signaling Pathway as an Anti-proliferation and Anti-metastatic Target in Hepatocarcinoma Cell Lines. J Cancer. 2020 Jan 1;11(2):432-440. doi: 10.7150/jca.30976. PMID: 31897238; PMCID: PMC6930431.
- Orme MH, Giannini AL, Vivanco MD, Kypta RM. Glycogen synthase kinase-3 and Axin function in a beta-catenin-independent pathway that regulates neurite outgrowth in neuroblastoma cells. Mol Cell Neurosci. 2003 Nov;24(3):673-86. doi: 10.1016/s1044-7431(03)00229-x. PMID: 14664817.
- Garan A, Akyüz S, Oztürk LK, Yarat A. Salivary parameters and caries indices in children with black tooth stains. J Clin Pediatr Dent. 2012 Spring;36(3):285-8. doi: 10.17796/jcpd.36.3.21466m672t723713. PMID: 22838232.
- Nusse R, Clevers H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell. 2017 Jun 1;169(6):985-999. doi: 10.1016/j.cell.2017.05.016. PMID: 28575679.
- Huang H, He X. Wnt/beta-catenin signaling: new (and old) players and new insights. Curr Opin Cell Biol. 2008 Apr;20(2):119-25. doi: 10.1016/j.ceb.2008.01.009. Epub 2008 Mar 12. PMID: 18339531; PMCID: PMC2390924.
- Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005 Jan-Mar;9(1):59-71. doi: 10.1111/j.1582-4934.2005.tb00337.x. PMID: 15784165; PMCID: PMC6741304.
- Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003 Apr 1;116(Pt 7):1175-86. doi: 10.1242/jcs.00384. PMID: 12615961; PMCID: PMC3006448.
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995 Dec 21-28;378(6559):785-9. doi: 10.1038/378785a0. PMID: 8524413.
- Krasilnikov MA. Phosphatidylinositol-3 kinase dependent pathways: the role in control of cell growth, survival, and malignant transformation. Biochemistry (Mosc). 2000 Jan;65(1):59-67. PMID: 10702641.
- Huang L, Fu L. Mechanisms of resistance to EGFR tyrosine kinase inhibitors. Acta Pharm Sin B. 2015 Sep;5(5):390-401. doi: 10.1016/j.apsb.2015.07.001. Epub 2015 Jul 26. PMID: 26579470; PMCID: PMC4629442.
- Torres MA, Eldar-Finkelman H, Krebs EG, Moon RT. Regulation of ribosomal S6 protein kinase-p90(rsk), glycogen synthase kinase 3, and beta-catenin in early Xenopus development. Mol Cell Biol. 1999 Feb;19(2):1427-37. doi: 10.1128/MCB.19.2.1427. PMID: 9891076; PMCID: PMC116071.
- Dailey L, Ambrosetti D, Mansukhani A, Basilico C. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev. 2005 Apr;16(2):233-47. doi: 10.1016/j.cytogfr.2005.01.007. Epub 2005 Mar 5. PMID: 15863038.
- Alcaraz E, Vilardell J, Borgo C, Sarró E, Plana M, Marin O, Pinna LA, Bayascas JR, Meseguer A, Salvi M, Itarte E, Ruzzene M. Effects of CK2β subunit down-regulation on Akt signalling in HK-2 renal cells. PLoS One. 2020 Jan 7;15(1):e0227340. doi: 10.1371/journal.pone.0227340. PMID: 31910234; PMCID: PMC6946142.
- Kennedy SG, Wagner AJ, Conzen SD, Jordán J, Bellacosa A, Tsichlis PN, Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997 Mar 15;11(6):701-13. doi: 10.1101/gad.11.6.701. PMID: 9087425.
- Hakim SG, Ribbat J, Berndt A, Richter P, Kosmehl H, Benedek GA, Jacobsen HC, Trenkle T, Sieg P, Rades D. Expression of Wnt-1, TGF-β and related cell-cell adhesion components following radiotherapy in salivary glands of patients with manifested radiogenic xerostomia. Radiother Oncol. 2011 Oct;101(1):93-9. doi: 10.1016/j.radonc.2011.07.032. Epub 2011 Aug 30. PMID: 21885141.
- Hai B, Yang Z, Shangguan L, Zhao Y, Boyer A, Liu F. Concurrent transient activation of Wnt/β-catenin pathway prevents radiation damage to salivary glands. Int J Radiat Oncol Biol Phys. 2012 May 1;83(1):e109-16. doi: 10.1016/j.ijrobp.2011.11.062. Epub 2012 Feb 16. PMID: 22342093; PMCID: PMC3340568.
- Haidar ZS. Bio-Inspired/-Functional Colloidal Core-Shell Polymeric-Based NanoSystems: Technology Promise in Tissue Engineering, Bioimaging and NanoMedicine. Polymers . 2010; 2: 323-352. https://doi.org/10.3390/polym2030323.
- Riley P, Glenny AM, Hua F, Worthington HV. Pharmacological interventions for preventing dry mouth and salivary gland dysfunction following radiotherapy. Cochrane Database Syst Rev. 2017 Jul 31;7(7):CD012744. doi: 10.1002/14651858.CD012744. PMID: 28759701; PMCID: PMC6483146.
- Ocampo J, Vásquez B, Sandoval C, Navarrete J, Haidar ZS, Olate, S. Características Morfocuantitativas de la Glándula Submandibular de Ratón (Mus musculus)/ Morphocuantitative Characteristics of the Mouse (Mus musculus) Submandibular Gland. International Journal of Morphology. 2020; 38: 570-577. https://dx.doi.org/10.4067/S0717-95022020000300570.
- Ocampo J, Olate S, Haidar ZS, Vásquez B, Hiposialia y .Xerostomía Post Irradiación: Terapias Innovadoras en el Campo Biomolecular/Hyposialia and Xerostomy Post Irradiation: Innovative Therapies in the Biomolecular Field. International Journal of Morphology 2019; 37: 1564-1571. https://dx.doi.org/10.4067/S0717-95022019000401564.
- Arany S, Xu Q, Hernady E, Benoit DS, Dewhurst W, Ovitt S, C. E. Pro-apoptotic gene knockdown mediated by nanocomplexed siRNA reduces radiation damage in primary salivary gland cultures. J. Cell. Biochem. 2012; 113, 1955–65, doi:10.1002/jcb.24064.
- Yoon YJ, Shin HS, Lim JY. A hepatocyte growth factor/MET-induced antiapoptotic pathway protects against radiation-induced salivary gland dysfunction. Radiother Oncol. 2019 Sep;138:9-16. doi: 10.1016/j.radonc.2019.05.012. Epub 2019 May 25. PMID: 31136962.
- Tai G, Zhang H, Du J, Chen G, Huang J, Yu J, Cai J, Liu F. TIGAR overexpression diminishes radiosensitivity of parotid gland fibroblast cells and inhibits IR-induced cell autophagy. Int J Clin Exp Pathol. 2015 May 1;8(5):4823-9. PMID: 26191173; PMCID: PMC4503045.
- Wie SM, Wellberg E, Karam SD, Reyland ME. Tyrosine Kinase Inhibitors Protect the Salivary Gland from Radiation Damage by Inhibiting Activation of Protein Kinase C-δ. Mol Cancer Ther. 2017 Sep;16(9):1989-1998. doi: 10.1158/1535-7163.MCT-17-0267. Epub 2017 Jun 21. PMID: 28637715; PMCID: PMC5587414.
- Choi JS, An HY, Shin HS, Kim YM, Lim JY. Enhanced tissue remodelling efficacy of adipose-derived mesenchymal stem cells using injectable matrices in radiation-damaged salivary gland model. J Tissue Eng Regen Med. 2018 Feb;12(2):e695-e706. doi: 10.1002/term.2352. Epub 2017 May 7. PMID: 27860388.
- Zeidan YH, Xiao N, Cao H, Kong C, Le QT, Sirjani D. Botulinum Toxin Confers Radioprotection in Murine Salivary Glands. Int J Radiat Oncol Biol Phys. 2016 Apr 1;94(5):1190-7. doi: 10.1016/j.ijrobp.2015.12.371. Epub 2015 Dec 29. PMID: 26907915; PMCID: PMC4839970.
- An HY, Shin HS, Choi JS, Kim HJ, Lim JY, Kim YM. Adipose Mesenchymal Stem Cell Secretome Modulated in Hypoxia for Remodeling of Radiation-Induced Salivary Gland Damage. PLoS One. 2015 Nov 3;10(11):e0141862. doi: 10.1371/journal.pone.0141862. PMID: 26529411; PMCID: PMC4631328.
- Xu L, Yang X, Cai J, Ma J, Cheng H, Zhao K, Yang L, Cao Y, Qin Q, Zhang C, Zhang Q, Sun X. Resveratrol attenuates radiation-induced salivary gland dysfunction in mice. Laryngoscope. 2013 Nov;123(11):E23-9. doi: 10.1002/lary.24276. Epub 2013 Jul 12. PMID: 23794219.
- Okumura H, Nasu M, Yosue T. Effects of amifostine administration prior to irradiation to the submandibular gland in mice: autoradiographic study using 3H-leucine. Okajimas Folia Anat Jpn. 2009 Feb;85(4):151-60. doi: 10.2535/ofaj.85.151. PMID: 19408584.
- Cotrim AP, Sowers A, Mitchell JB, Baum BJ. Prevention of irradiation-induced salivary hypofunction by microvessel protection in mouse salivary glands. Mol Ther. 2007 Dec;15(12):2101-6. doi: 10.1038/sj.mt.6300296. Epub 2007 Aug 28. PMID: 17726456.
- Cotrim AP, Hyodo F, Matsumoto K, Sowers AL, Cook JA, Baum BJ, Krishna MC, Mitchell JB. Differential radiation protection of salivary glands versus tumor by Tempol with accompanying tissue assessment of Tempol by magnetic resonance imaging. Clin Cancer Res. 2007 Aug 15;13(16):4928-33. doi: 10.1158/1078-0432.CCR-07-0662. PMID: 17699873.
- Cotrim AP, Sowers AL, Lodde BM, Vitolo JM, Kingman A, Russo A, Mitchell JB, Baum BJ. Kinetics of tempol for prevention of xerostomia following head and neck irradiation in a mouse model. Clin Cancer Res. 2005 Oct 15;11(20):7564-8. doi: 10.1158/1078-0432.CCR-05-0958. PMID: 16243832.
- Aonuma M, Nasu M, Iwata H, Yosue T. Radioprotection of the murine submandibular gland by isoproterenol: autoradiography study with 3H-leucine. Odontology. 2004 Sep;92(1):14-21. doi: 10.1007/s10266-004-0032-7. PMID: 15490300.
- Vitolo JM, Cotrim AP, Sowers AL, Russo A, Wellner RB, Pillemer SR, Mitchell JB, Baum BJ. The stable nitroxide tempol facilitates salivary gland protection during head and neck irradiation in a mouse model. Clin Cancer Res. 2004 Mar 1;10(5):1807-12. doi: 10.1158/1078-0432.ccr-03-0194. PMID: 15014035.
- Rasey JS, Krohn KA, Menard TW, Spence AM. Comparative biodistribution and radioprotection studies with three radioprotective drugs in mouse tumors. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1487-90. doi: 10.1016/0360-3016(86)90200-2. PMID: 3019965.
- Rasey JS, Nelson NJ, Mahler P, Anderson K, Krohn KA, Menard T. Radioprotection of normal tissues against gamma rays and cyclotron neutrons with WR-2721: LD50 studies and 35S-WR-2721 biodistribution. Radiat Res. 1984 Mar;97(3):598-607. PMID: 6328565.
- Varghese JJ, Schmale IL, Mickelsen D, Hansen ME, Newlands SD, Benoit DSW, Korshunov VA, Ovitt CE. Localized Delivery of Amifostine Enhances Salivary Gland Radioprotection. J Dent Res. 2018 Oct;97(11):1252-1259. doi: 10.1177/0022034518767408. Epub 2018 Apr 10. Erratum in: J Dent Res. 2018 Aug;97(9):1070. PMID: 29634396; PMCID: PMC6151913.
- Shin HS, Lee S, Kim YM, Lim JY. Hypoxia-Activated Adipose Mesenchymal Stem Cells Prevents Irradiation-Induced Salivary Hypofunction by Enhanced Paracrine Effect Through Fibroblast Growth Factor 10. Stem Cells. 2018 Jul;36(7):1020-1032. doi: 10.1002/stem.2818. Epub 2018 Apr 10. PMID: 29569790.
- Toshkov IA, Gleiberman AS, Mett VL, Hutson AD, Singh AK, Gudkov AV, Burdelya LG. Mitigation of Radiation-Induced Epithelial Damage by the TLR5 Agonist Entolimod in a Mouse Model of Fractionated Head and Neck Irradiation. Radiat Res. 2017 May;187(5):570-580. doi: 10.1667/RR14514.1. Epub 2017 Mar 21. PMID: 28323577; PMCID: PMC5541767.
- Xu L, Yang X, Chen J, Ge X, Qin Q, Zhu H, Zhang C, Sun X. Simvastatin attenuates radiation-induced salivary gland dysfunction in mice. Drug Des Devel Ther. 2016 Jul 13;10:2271-8. doi: 10.2147/DDDT.S105809. PMID: 27471375; PMCID: PMC4948692.
- Crescenti EJ, Medina VA, Croci M, Sambuco LA, Prestifilippo JP, Elverdin JC, Bergoc RM, Rivera ES. Radioprotection of sensitive rat tissues by oligoelements Se, Zn, Mn plus Lachesis muta venom. J Radiat Res. 2011;52(5):557-67. doi: 10.1269/jrr.11031. PMID: 21952314.
- Coppes RP, Vissink A, Zeilstra LJ, Konings AW. Muscarinic receptor stimulation increases tolerance of rat salivary gland function to radiation damage. Int J Radiat Biol. 1997 Nov;72(5):615-25. doi: 10.1080/095530097143112. PMID: 9374441.
- Coppes RP, Zeilstra LJ, Vissink A, Konings AW. Sialogogue-related radioprotection of salivary gland function: the degranulation concept revisited. Radiat Res. 1997 Sep;148(3):240-7. PMID: 9291355.
- Menard TW, Izutsu KT, Ensign WY, Keller PJ, Morton TH, Truelove EL. Radioprotection by WR-2721 of gamma-irradiated rat parotid gland: effect on gland weight and secretion at 8-10 days post irradiation. Int J Radiat Oncol Biol Phys. 1984 Sep;10(9):1555-9. doi: 10.1016/0360-3016(84)90502-9. PMID: 6090361.
- Sodicoff M, Conger AD. Radioprotection of the rat parotid gland by cAMP. Radiat Res. 1983 Oct;96(1):90-4. PMID: 6312484.
- Sodicoff M, Conger AD. Radioprotection of the rat parotid gland by WR-2721 and isoproterenol and its modification by propranolol. Radiat Res. 1983 Apr;94(1):97-104. PMID: 6304806.
- Pratt NE, Sodicoff M, Liss J, Davis M, Sinesi M. Radioprotection of the rat parotid gland by WR-2721: morphology at 60 days post-irradiation. Int J Radiat Oncol Biol Phys. 1980 Apr;6(4):431-5. doi: 10.1016/0360-3016(80)90056-5. PMID: 6248498.
- Sodicoff M, Conger AD, Pratt NE, Trepper P. Radioprotection by WR-2721 against long-term chronic damage to the rat parotid gland. Radiat Res. 1978 Oct;76(1):172-9. PMID: 216048.
- Abedi SM, Yarmand F, Motallebnejad M, Seyedmajidi M, Moslemi D, Bijani A, Hosseinimehr SJ. Radioprotective Effect of Thymol Against Salivary Glands Dysfunction Induced by Ionizing Radiation in Rats. Iran J Pharm Res. 2016 Fall;15(4):861-866. PMID: 28243283; PMCID: PMC5316265.
- Palaniyandi S, Odaka Y, Green W, Abreo F, Caldito G, De Benedetti A, Sunavala-Dossabhoy G. Adenoviral delivery of Tousled kinase for the protection of salivary glands against ionizing radiation damage. Gene Ther. 2011 Mar;18(3):275-82. doi: 10.1038/gt.2010.142. Epub 2010 Nov 4. PMID: 21048794.
- Timiri Shanmugam PS, Dayton RD, Palaniyandi S, Abreo F, Caldito G, Klein RL, Sunavala-Dossabhoy G. Recombinant AAV9-TLK1B administration ameliorates fractionated radiation-induced xerostomia. Hum Gene Ther. 2013 Jun;24(6):604-12. doi: 10.1089/hum.2012.235. PMID: 23614651; PMCID: PMC3689188.
- Hakim SG, Benedek GA, Su YX, Jacobsen HC, Klinger M, Dendorfer A, Hemmelmann C, Meller B, Nadrowitz R, Rades D, Sieg P. Radioprotective effect of lidocaine on function and ultrastructure of salivary glands receiving fractionated radiation. Int J Radiat Oncol Biol Phys. 2012 Mar 15;82(4):e623-30. doi: 10.1016/j.ijrobp.2011.09.017. Epub 2012 Jan 13. PMID: 22245203.
- Hakim SG, Kosmehl H, Lauer I, Nadrowitz R, Wedel T, Sieg P. A comparative study on the protection profile of lidocaine, amifostine, and pilocarpin on the parotid gland during radiotherapy. Cancer Res. 2005 Nov 15;65(22):10486-93. doi: 10.1158/0008-5472.CAN-05-0023. PMID: 16288041.
- Lotz S, Caselitz J, Tschakert H, Rehpenning W, Seifert G. Radioprotection of minipig salivary glands by orciprenaline-carbachol. An ultrastructural and semiquantitative light microscopic study. Virchows Arch A Pathol Anat Histopathol. 1990;417(2):119-28. doi: 10.1007/BF02190529. PMID: 2114690.
- Guo L, Gao R, Xu J, Jin L, Cotrim AP, Yan X, Zheng C, Goldsmith CM, Shan Z, Hai B, Zhou J, Zhang C, Baum BJ, Wang S. AdLTR2EF1α-FGF2-mediated prevention of fractionated irradiation-induced salivary hypofunction in swine. Gene Ther. 2014 Oct;21(10):866-73. doi: 10.1038/gt.2014.63. Epub 2014 Jul 17. PMID: 25030610.
- McDonald S, Meyerowitz C, Smudzin T, Rubin P. Preliminary results of a pilot study using WR-2721 before fractionated irradiation of the head and neck to reduce salivary gland dysfunction. Int J Radiat Oncol Biol Phys. 1994 Jul 1;29(4):747-54. doi: 10.1016/0360-3016(94)90562-2. PMID: 8040020.
- Baum RP, Langbein T, Singh A, Shahinfar M, Schuchardt C, Volk GF, Kulkarni H. Injection of Botulinum Toxin for Preventing Salivary Gland Toxicity after PSMA Radioligand Therapy: an Empirical Proof of a Promising Concept. Nucl Med Mol Imaging. 2018 Feb;52(1):80-81. doi: 10.1007/s13139-017-0508-3. Epub 2018 Jan 11. PMID: 29391917; PMCID: PMC5777965.
- Vacha P, Fehlauer F, Mahlmann B, Marx M, Hinke A, Sommer K, Richter E, Feyerabend T. Randomized phase III trial of postoperative radiochemotherapy +/- amifostine in head and neck cancer. Is there evidence for radioprotection? Strahlenther Onkol. 2003 Jun;179(6):385-9. doi: 10.1007/s00066-003-1016-1. PMID: 12789464.
- Scrimger RA, Seikaly H, Vos LJ, Harris J, O'Connell D, Ghosh S, Debenham B, Jha N. Combination of submandibular salivary gland transfer and intensity-modulated radiotherapy to reduce dryness of mouth (xerostomia) in patients with head and neck cancer. Head Neck. 2018 Nov;40(11):2353-2361. doi: 10.1002/hed.25339. Epub 2018 Sep 3. PMID: 30175876.
- Teng F, Fan W, Luo Y, Ju Z, Gong H, Ge R, Tong F, Zhang X, Ma L. Reducing Xerostomia by Comprehensive Protection of Salivary Glands in Intensity-Modulated Radiation Therapy with Helical Tomotherapy Technique for Head-and-Neck Cancer Patients: A Prospective Observational Study. Biomed Res Int. 2019 Jul 14;2019:2401743. doi: 10.1155/2019/2401743. PMID: 31380414; PMCID: PMC6662416.
- Saito E, Watari I, Mizumachi-Kubono M, Hsu-Hayashi S, Ono T. Occlusional Modifications Reversibly Alter Aquaporin 5 Expression and Localization in Rat Salivary Glands. Front Physiol. 2020 Jun 10;11:528. doi: 10.3389/fphys.2020.00528. PMID: 32587522; PMCID: PMC7298139.
- Liu X, Gong B, de Souza LB, Ong HL, Subedi KP, Cheng KT, Swaim W, Zheng C, Mori Y, Ambudkar IS. Radiation inhibits salivary gland function by promoting STIM1 cleavage by caspase-3 and loss of SOCE through a TRPM2-dependent pathway. Sci Signal. 2017 Jun 6;10(482):eaal4064. doi: 10.1126/scisignal.aal4064. PMID: 28588080; PMCID: PMC5798857.
- Wang TS, Coppens I, Saorin A, Brady NR, Hamacher-Brady A. Endolysosomal Targeting of Mitochondria Is Integral to BAX-Mediated Mitochondrial Permeabilization during Apoptosis Signaling. Dev Cell. 2020 Jun 22;53(6):627-645.e7. doi: 10.1016/j.devcel.2020.05.014. Epub 2020 Jun 5. PMID: 32504557; PMCID: PMC7433306.
- Marmary Y, Adar R, Gaska S, Wygoda A, Maly A, Cohen J, Eliashar R, Mizrachi L, Orfaig-Geva C, Baum BJ, Rose-John S, Galun E, Axelrod JH. Radiation-Induced Loss of Salivary Gland Function Is Driven by Cellular Senescence and Prevented by IL6 Modulation. Cancer Res. 2016 Mar 1;76(5):1170-80. doi: 10.1158/0008-5472.CAN-15-1671. Epub 2016 Jan 12. Erratum in: Cancer Res. 2016 May 1;76(9):2846. PMID: 26759233.
- Adwan TS, Ohm AM, Jones DNM, Humphries MJ, Reyland ME. Regulated binding of importin-α to protein kinase Cδ in response to apoptotic signals facilitates nuclear import. J Biol Chem. 2011 Oct 14;286(41):35716-35724. doi: 10.1074/jbc.M111.255950. Epub 2011 Aug 24. PMID: 21865164; PMCID: PMC3195609.
- van Dijk LV, Fuller CD. Artificial Intelligence and Radiomics in Head and Neck Cancer Care: Opportunities, Mechanics, and Challenges. Am Soc Clin Oncol Educ Book. 2021 Mar;41:1-11. doi: 10.1200/EDBK_320951. PMID: 33929877; PMCID: PMC8218312.
- Maleki F, Le WT, Sananmuang T, Kadoury S, Forghani R. Machine Learning Applications for Head and Neck Imaging. Neuroimaging Clin N Am. 2020 Nov;30(4):517-529. doi: 10.1016/j.nic.2020.08.003. PMID: 33039001.
- Chinnery T, Arifin A, Tay KY, Leung A, Nichols AC, Palma DA, Mattonen SA, Lang P. Utilizing Artificial Intelligence for Head and Neck Cancer Outcomes Prediction From Imaging. Can Assoc Radiol J. 2021 Feb;72(1):73-85. doi: 10.1177/0846537120942134. Epub 2020 Jul 31. PMID: 32735452.
- Mahmood H, Shaban M, Rajpoot N, Khurram SA. Artificial Intelligence-based methods in head and neck cancer diagnosis: an overview. Br J Cancer. 2021 Jun;124(12):1934-1940. doi: 10.1038/s41416-021-01386-x. Epub 2021 Apr 19. PMID: 33875821; PMCID: PMC8184820.
- Abdel Razek AAK, Khaled R, Helmy E, Naglah A, AbdelKhalek A, El-Baz A. Artificial Intelligence and Deep Learning of Head and Neck Cancer. Magn Reson Imaging Clin N Am. 2022 Feb;30(1):81-94. doi: 10.1016/j.mric.2021.06.016. PMID: 34802583.