More Information
Submitted: February 07, 2023 | Approved: February 22, 2023 | Published: February 23, 2023
How to cite this article: Li Y, Liu H, Ding L, You L, Zhang Y, et al. Effects of Pleiotrophin (PTN) on the resistance to paclitaxel in ovarian cancer cells. J Radiol Oncol. 2023; 7: 006-012.
DOI: 10.29328/journal.jro.1001046
Copyright License: © 2023 Li Y, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Effects of Pleiotrophin (PTN) on the resistance to paclitaxel in ovarian cancer cells
Yunfei Li#, Huali Liu#, Linlin Ding, Liwei You, Yuqiang Zhang, Xingxing Wang, Xueyuan Lin and Liquan Yang*
The Center for Combinatorial Chemistry and Drug Discovery, The School of Pharmaceutical Sciences, Jilin University, Changchun, Jilin, People’s Republic of China
#Contributed equally to the writing of this article
*Address for Correspondence: Liquan Yang, Ph.D, The Center for Combinatorial Chemistry and Drug Discovery, The School of Pharmaceutical Sciences, Jilin University, 1266 Fujin Road, Changchun, Jilin 130021, People’s Republic of China, Email: [email protected]
The pathogenesis of an ovarian disease is connected with PTN and its receptor protein tyrosine phosphatase receptor Z1 (PTPRZ1). Paclitaxel is the first-line drug for the therapy of ovarian cancer. With the increment of paclitaxel chemotherapy, paclitaxel obstruction happens in the late phase of therapy frequently. By treating A2780 and SKOV-3 cells with PTN, we found the development of the two cell lines was enhanced. Different concentrations of PTN were added to A2780 and SKOV-3 cells treated with paclitaxel and the results of MTT showed that the inhibitory effect of paclitaxel on these two cell lines was weakened. The results of apoptosis assays showed that PTN could slow down the rate of apoptosis and its concentration dependence in both cell lines. To further investigate the impact of PTN on the paclitaxel responsiveness of ovarian malignant growth cells, A2780 and SKOV-3 cells were transfected with sh-PTN-1, sh-PTN-2 and sh-NC plasmids. The results of PCR and Western Blot showed that both RNA-interfering plasmids could inhibit PTN in A2780 and SKOV-3 cells. The results of MTT showed that the inhibitory effect of paclitaxel on cells transfected with sh-PTN-1 expanded compared with the benchmark group. Apoptosis assays showed that the complete apoptosis pace of A2780 and SKOV-3 cells with sh-PTN-1 plasmid induced by paclitaxel was accelerated obviously compared with the benchmark group. To summarize, the results suggested that PTN could enhance the resistance to paclitaxel in ovarian cancer cells, which provides a groundwork for studying on drug resistance of cancer cells to paclitaxel and a new perspective for ovarian cancer therapy.
One of the most prevalent malignant tumors of the female reproductive system, ovarian cancer has both a high incidence and fatality rate [1]. It saw 314 thousand new tumor cases, with 55 thousand of those in China, which accounted for 17% of all cases in 2020. Additionally, there were 207 thousand of new fatalities, with 37 thousand of those in China, which accounted for 18% [2]. Ovarian cancer is characterized by a high incidence rate and mortality, and the incidence rate is increasing every year [3]. A serious concealed hazard to women’s health is ovarian cancer. PTN is expressed prominently in a variety of malignancies, including ovarian, rectal, pancreatic, lung, prostate, and skin cancers [4]. PTN is crucial for the growth and spread of cancer cells, making it a potential biomarker or target for the development of new anticancer drugs [5]. Paclitaxel is a first-line drug for the treatment of ovarian cancer. It has the effect of treating tumors by promoting the assembly and stability of tubulin [6]. For most ovarian cancer patients, in the beginning paclitaxel chemotherapy has good efficacy. But paclitaxel resistance, a significant factor leading to patients’ death, frequently occurs in later stages of treatments. PTN, on the other hand, is highly expressed in ovarian cancer cells. studies indicate that PTN and its receptor protein tyrosine phosphatase receptor Z1 (PTPRZ1) may be involved in the pathogenesis of ovarian cancer [7,8]. The study aims to investigate whether PTN is linked to paclitaxel resistance in ovarian cancer and to create a new approach to ovarian cancer treatment.
Cell culture
Jilin University’s Basic Medical College provided the Human ovarian cancer cell lines SKOV-3. Human ovarian cancer cells A2780 were obtained from Jilin University’s Research Institute of Translational Medicine. The A2780 and SKOV-3 cells were removed from the liquid nitrogen, quickly transferred to a water bath preheated up to 37 ℃, and gently shaken for 1~2 min to melt. Cells were transferred to a centrifuge tube in a clean bench, centrifuged at 1200 rpm at 3 min and the supernatant was aspirated. The precipitate was resuspended with RPMI 1640 medium in 10% FBS and the cells were transferred to a 37 ℃, 5% CO2 incubator [9].
Effect of PTN on the growth of A2780 and SKOV-3 cells
The exogenous pleiotrophin was obtained and purified in our laboratory using a pleiotrophin recombinant plasmid. Cells of A2780 and SKOV-3 in the log-growth phase were seeded in a 24-well plate at a density of 5 × 104, and the pleiotrophin was added at the concentration of 0,10,20,50,100,150,200 ng/ml the next day. After 48 h, the cells were counted to draw the cell growth curve.
Effect of PTN on A2780 and SKOV-3 cell growth curve
A2780 and SKOV-3 in the logarithmic growth phase were seeded into 24-well plates at a density of 2 × 104 cells/well. On the second day it was processed with different PTN concentrations at 0,10,20,50,100,150,200 ng/ml. After 48 hours, cell growth curves were drawn with the PTN concentration as an abscissa and the cell number as an ordinate.
Effect of PTN on paclitaxel sensitivity in A2780 and SKOV-3 cells
Cells were resuspended in a culture medium, and the cell density was adjusted to 5 × 104/ml. Control holes and zero-adjusting holes were installed, as well as three compound holes were set for each group. Control wells were not drug treated and the zero adjustment hole was supplemented with 100 ml of medium. Then 0,10,20,40,60,80,100 μM paclitaxel was added after 24 hours. The 100 ng/ml PTN was added to the other group again. The absorption value at 490 nm was measured by a microplate reader and the mean of each concentration was calculated. To observe the rate of cell inhibition by paclitaxel after the addition of PTN, the data was processed with Excel and GraphPad to calculate IC50 [10,11].
Effect of PTN on apoptosis in A2780 and SKOV-3 cells
The cells were divided into five groups, one of which serves as a control group without treatment. The other four groups were treated with paclitaxel and 0 ng/ml, 20 ng/ml, 50 ng/ml, and 100 ng/ml PTN protein followed by Annexin V-PI staining after 48 hours of incubation. Groups of data were observed and analyzed.
Effect of sh-PTN transfection on PTN mRNA
The A2780 and SKOV-3 cells were transfected at a density of about 90%. One group was not treated as a control, while the other three groups were transfected with the sh-NC (negative control), sh-PTN-1, and sh-PTN-2 plasmids [12]. After 24 h of the transfection, the fluorescence was visualized by fluorescence microscopy.
Western blotting
The cells were washed with cold PBS buffer, mixed with RIPA Lysis Buffer and PMSF, added to cell culture dishes, lysed on ice for 30 min, blown, and mixed continuously [13]. After the lysis, the mixture was transferred to a clean up tube via pipette and was centrifuged at 12000 r at 4 ℃ for 5 min. The supernatant was transferred to a new centrifuge tube, added for 5* Budder, boiled at 100 ℃ for 5 min, and cooled in an ice bath [14].
Protein concentration was measured by BCA: Mix fluids A and B in a ratio of 50:1 and set aside. The standard solution of BSA protein was diluted into a series of concentrations according to the instructions. Then the sample was diluted appropriately. The PBS of the wells of the BSA series was supplied to 20 μl. The 200 μl BCA working fluid was added to each well followed by incubation at 37 ℃ for 30 min. Then the absorbance value of 562 nm was detected with a microplate reader. The prepared PTN was used as the primary antibody at a 1:1,000 dilution. The data were processed by Image J and GraphPad analysis.
Effect of PTN silencing on paclitaxel sensitivity in A2780 and SKOV-3 cells
The A2780 and SKOV-3 cell density was adjusted to 5 × 104/ml. Three compound holes were set, and the control holes and zero-adjusting holes were set. The cells were cultured in an incubator at 37 ℃ and transfected after 24 h.
The sh-PTN-1 and sh-NC plasmids were transfected respectively. Transfection was visualized by fluorescence microscope after 24 h. Paclitaxel with the same concentration gradient was added after 24 h. Absorption values at 490 nm were detected by a microplate reader. Graphs were drawn and IC50 was calculated to observe the changes in cell inhibition rate after sh-PTN transfection [15].
Effect of PTN silencing on the apoptosis in paclitaxel-acting A2780 and SKOV-3 cells
Cells were seeded into 6-well plates and divided into the Control, sh-NC, and sh-PTN-1 groups. At 24 h after transfection, each well from the Control group, the sh-NC group, and the sh-PTN-1 group was taken to add the IC50 concentration of paclitaxel. All wells were stained after 24 h incubation.
The Cleaved PARP expression was measured in paclitaxel-acting PTN-silenced A2780 and SKOV-3 cells
The A2780 and SKOV-3 cells were cultured to the appropriate density for transfection. It is divided into two groups: the sh-PTN-1 group and the Control group. At 24 h after transfection, each well of the transfection and Control groups was taken to add IC50 concentration of paclitaxel leaving other wells untreated. The method is the same as the previous one. The expression of Cleaved PARP apoptotic protein was detected in each group of samples, and the β-actin was selected as the internal reference. The bands were analyzed for gray values by Image J, and the charts were treated with GraphPad.
Effect of PTN on the growth of A2780 and SKOV-3 cells
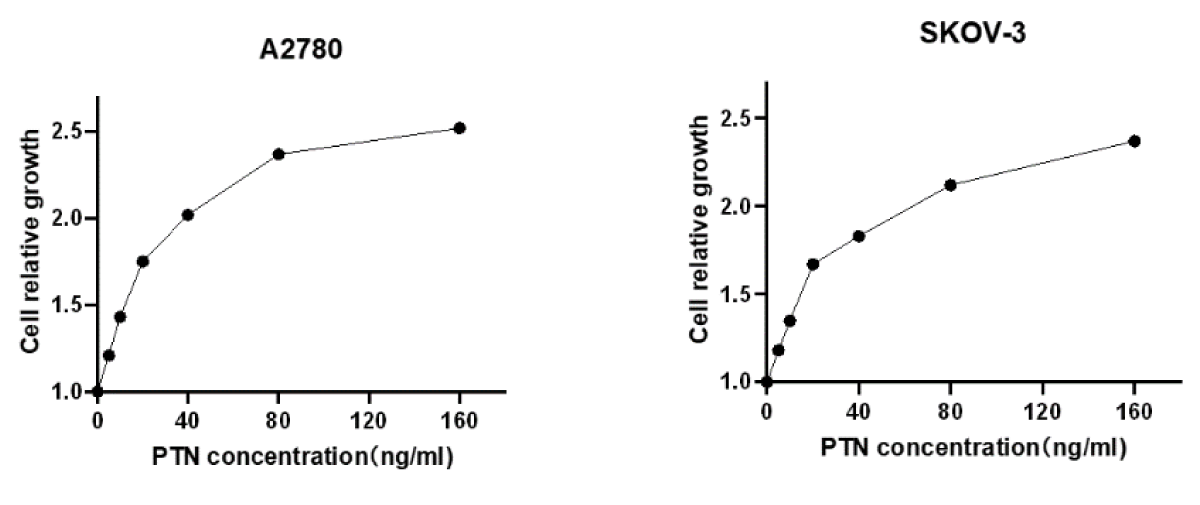
To verify whether exogenous PTN protein has an effect on cell growth, 2 × 104 A2780 and SKOV-3 cells were seeded in 24-well plates, and a gradient concentration of PTN protein was added the next day, and cells were counted 48 h later, with the relative number of cells as ordinate and PTN concentration as abscissa, as shown in Figure 3.4. According to the figure, the relative number of A2780 and SKOV-3 cells increased with the concentration of the PTN, showing a concentration-dependent growth, indicating that the exogenous PTN protein promoted the growth of A2780 and SKOV-3 cells (Figure 1).
Figure 1: The exogenous PTN protein promoted the growth of A2780 and SKOV-3 cells. The pleiotrophin was added at the concentration of 0,5,10,20,50,100,150 ng/ml. With the increase in the concentration of exogenous PTN protein, the cell growth rate increased.
Effect of PTN on paclitaxel sensitivity in A2780 and SKOV-3 cells
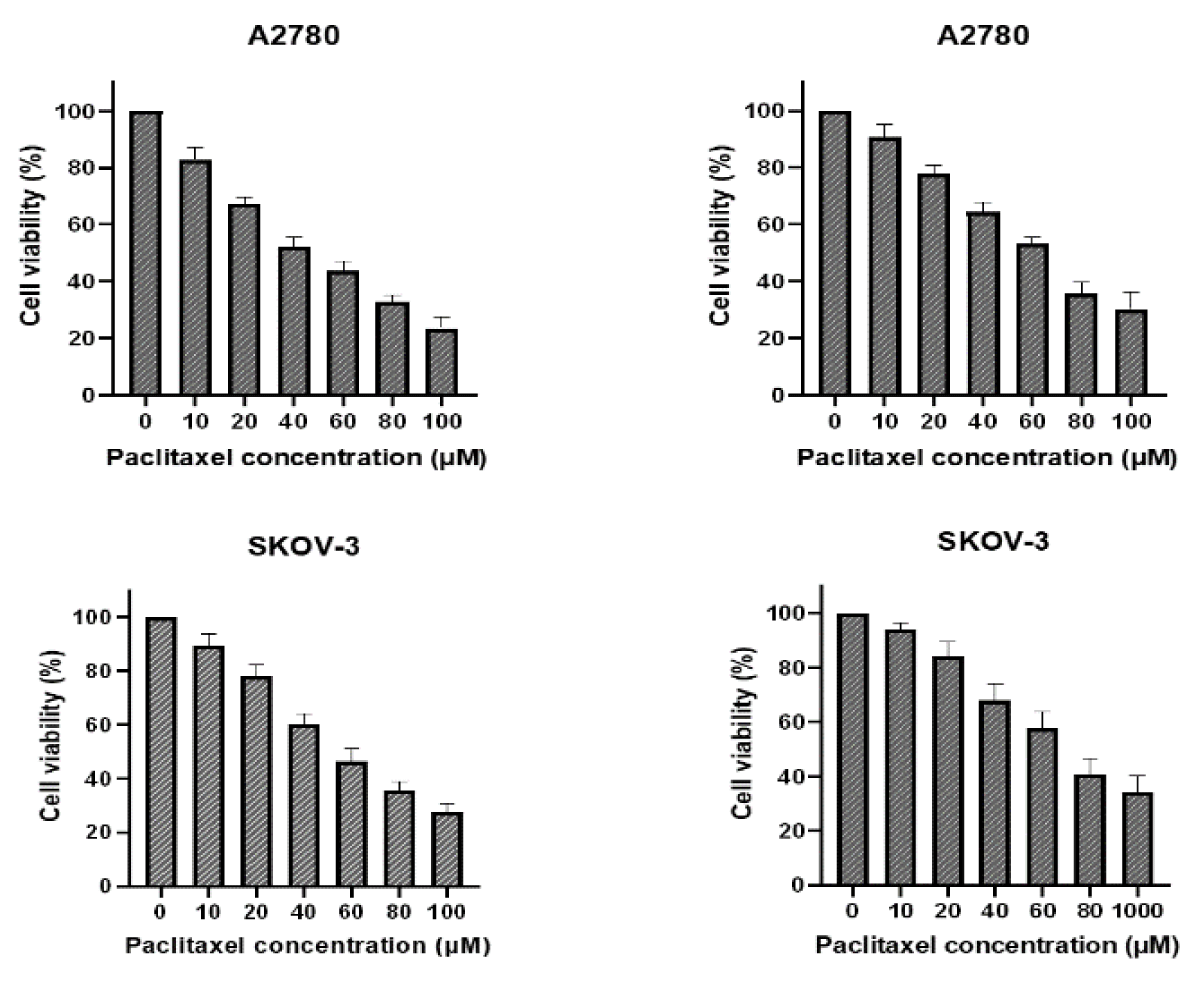
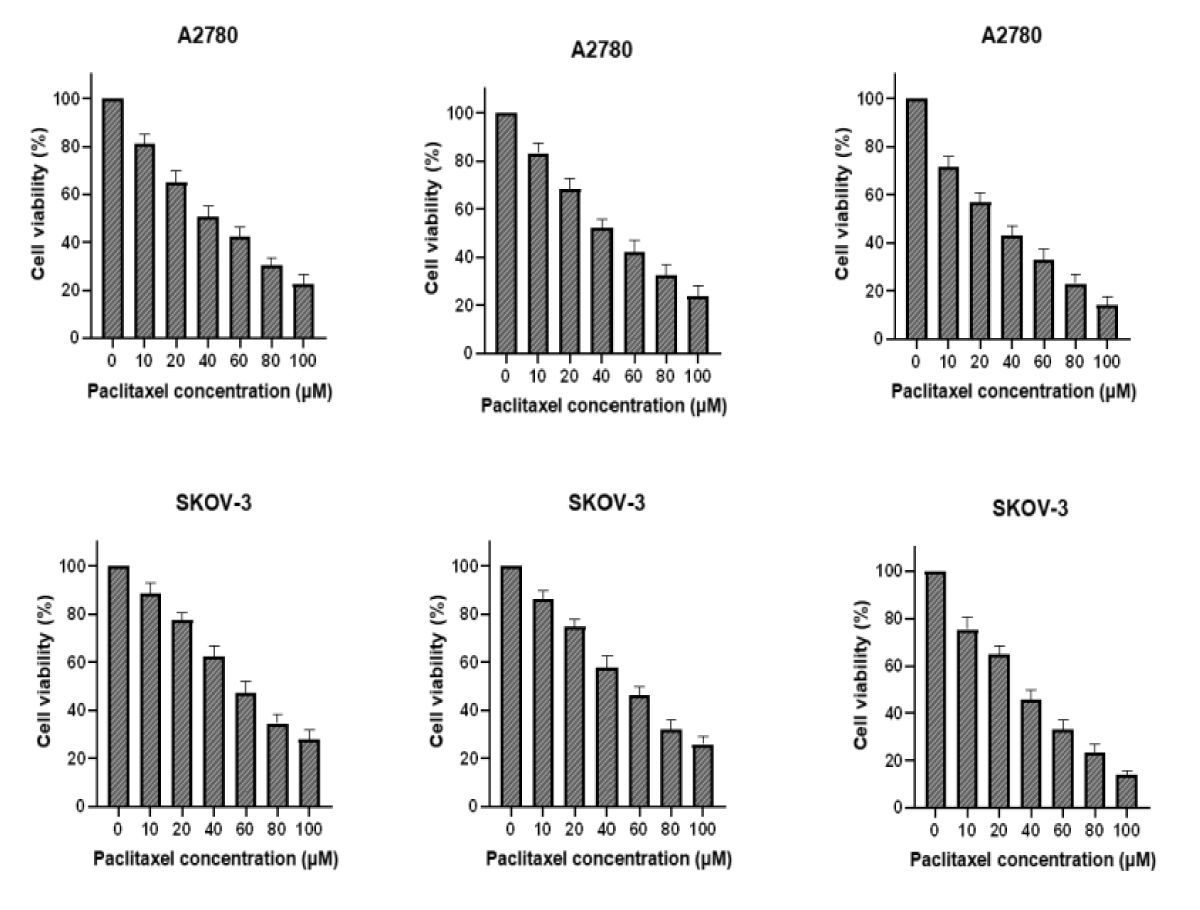
Spread 5 × 103 cells per well in 96-well plates, setting 6 concentrations and 3 compound wells. Paclitaxel was added to one plate the next day, and PTN protein was added in addition to the same concentration of paclitaxel. After 48 h, with OD490, cell survival was calculated and plotted, and IC50 was calculated by GraphPad (Figure 2).
Figure 2: A and C were paclitaxel; B and D were paclitaxel and PTN.As can be seen from the figure, the higher survival rate of the cells in the PTN group at the same drug concentration than that without the PTN group indicates that PTN can attenuate the killing effect of paclitaxel on A2780 and SKOV-3 cells, and reduce the sensitivity of A2780 and SKOV-3 cells to paclitaxel. The IC50 values for A2780 and SKOV-3 cells were 25.32 M and 29.73uM, respectively.
Effect of PTN on apoptosis of A2780 and SKOV-3 cells
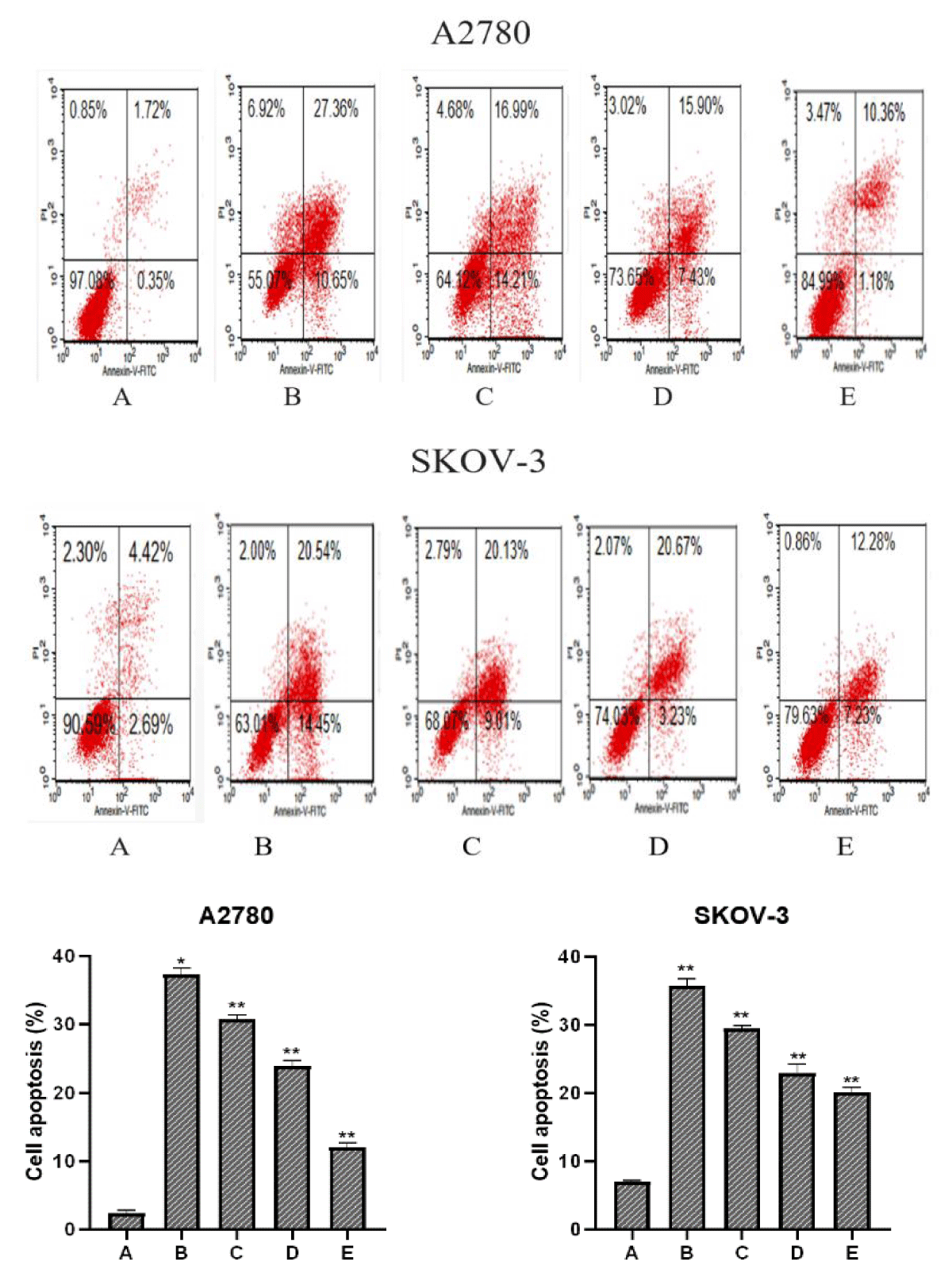
To explore whether PTN protein concentration affected the apoptotic effect of paclitaxel on A2780 and SKOV-3 cells, A2780 and SKOV-3 cells were divided into five groups, and group Control was treated without paclitaxel and PTN, IC50 of paclitaxel, then 0 ng/ml, 20 ng/ml, 50 ng/ml, 100 ng/ml of PTN and the apoptosis rate was detected after 48 h (Figure 3).
Figure 3: A, B, C, D and E indicate the Control group, paclitaxel + PTN (0 ng/ml) group, paclitaxel + PTN (20 ng/ml) group, paclitaxel + PTN (50 ng/ml) group, and paclitaxel + PTN (100 ng/ml) group, respectively. In comparison with the Control group, *p (0.05, **p ≤ 0.01). The early and late total apoptosis rates of two cell lines control group, paclitaxel + PTN (0ng/ml) group, paclitaxel + PTN (20 ng/ml) group, paclitaxel + PTN (50 ng/ml) group, paclitaxel + PTN (100 ng/ml) group were: A2780: 2.07%, 38.01%, 31.20%, 23.33%, 19.51%; SKOV-3: 4.35%, 34.96%, 29.14%, 23.90%, 14.56%, It can be seen from the figure that with the increase in the concentration of PTN, the rate of apoptosis decreases. The concentration of PTN and the rate of apoptosis show a concentration dependence and the results are statistically significant.
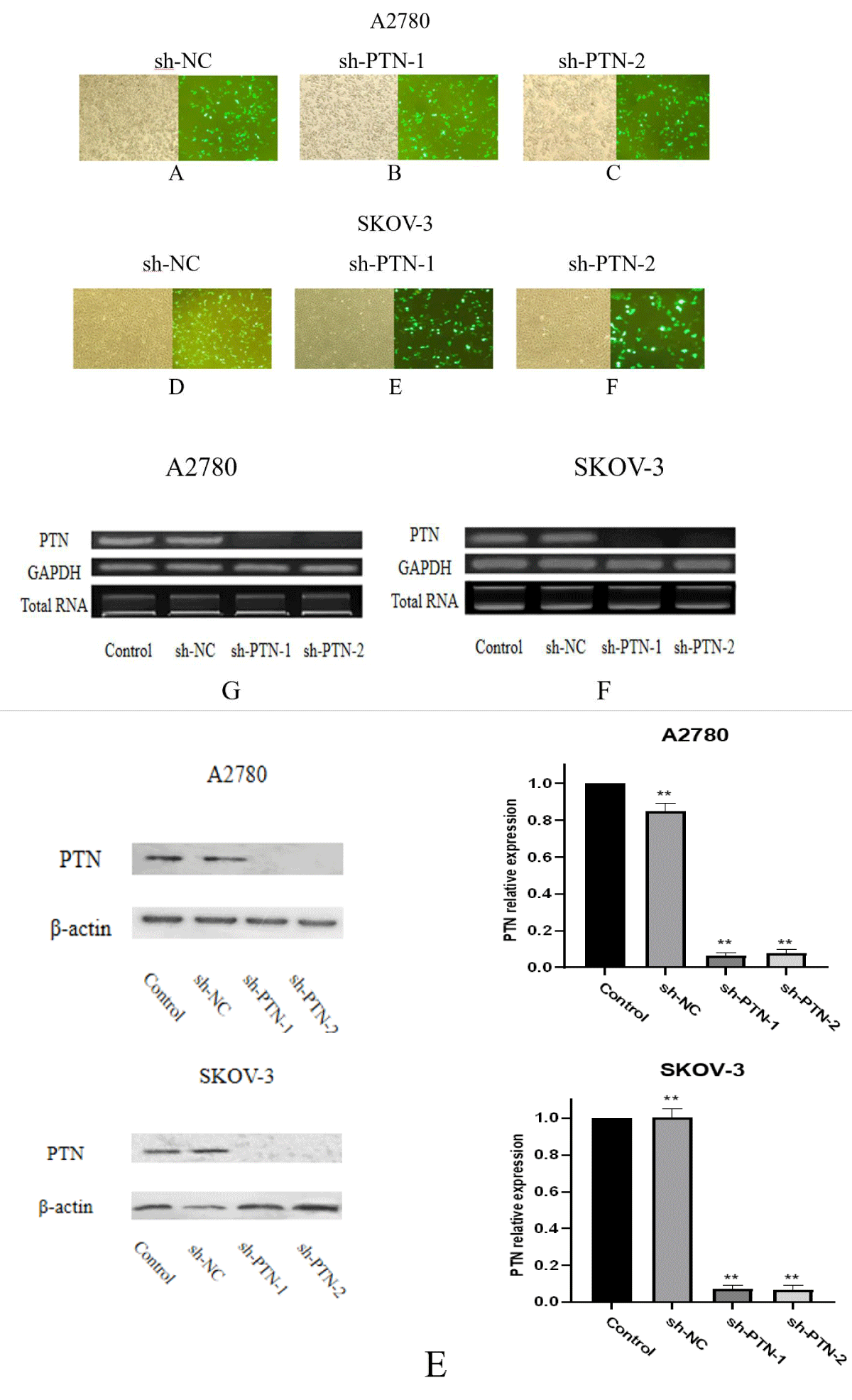
Effects of sh-PTN transfection on PTN mRNA and protein expression Sh-NC, sh-PTN-1, and sh-PTN-2 plasmids were transfected into A2780 and SKOV-3 cells, 24 hours after transfection, and the transfection results were observed under a fluorescence microscope. In order to explore the expression of PTN mRNA in A2780 and SKOV-3 cells after sh-PTN plasmid transfection, 24 hours after transfection, fluorescence was observed, total RNA was extracted and cDNA was obtained by reverse transcription. The gene was amplified by PCR using cDNA as a template and GAPDH as an internal reference, and the expression of PTN mRNA gene level in each transfection group and control group were compared (Figure 4).
Figure 4: Number of A-F shows the transfection observed in the fluorescent inverted microscope. a number of G, F shows the mRNA expression observed by PCR. E shows the amount of PTN expression after adding interference plasmid in SKOV-3 and A2780.
It can be seen from the figure that when the expression amount of internal reference GAPDH is the same, there is no significant difference in PTN mRNA expression between Control and sh-NC groups in the two cell lines, while the expression amount of PTN in sh-PTN-1 and sh-PTN-2 groups is reduced significantly. In A2780 and SKOV-3 cells, both sh-PTN plasmids play a significant interference role. During protein expression, In the two cell lines, there was no significant difference in the expression of PTN protein between the control group and the sh-NC group, but the expression of PTN protein in the sh-PTN-1 and sh-PTN-2 transfected groups was significantly lower than that in the control group. Both sh-PTN plasmids can play a good interference role.
MTT assay was used to detect the effect of PTN silencing on paclitaxel sensitivity of A2780 and SKOV-3 cells
A2780 and SKOV-3 cells were laid on 96 well plates and transfected with sh-NC plasmid and sh-PTN-1 plasmid. After 24 hours, paclitaxel with the same concentration gradient was added. After 48 hours, OD490 was measured to calculate the cell survival rate and draw a chart (Figure 5).
Figure 5: A2780 and SKOV-3 cells were transfected with sh-NC plasmid and sh-PTN-1 plasmid, then paclitaxel with the same concentration gradient was added and OD490 was determined.
The survival rates of the control group and the SH-NC group of the two cell lines at various concentrations were not significantly different, but the survival rates of the sh-pin-1 group at various paclitaxel concentrations were lower than those of the control group and the sh-NC group, that is, the inhibition of paclitaxel at the same concentration on the sh-pin-1 group was higher than that of the control group and the sh-NC group. After PTN expression was silenced, the sensitivity of A2780 and SKOV-3 cells to paclitaxel increased.
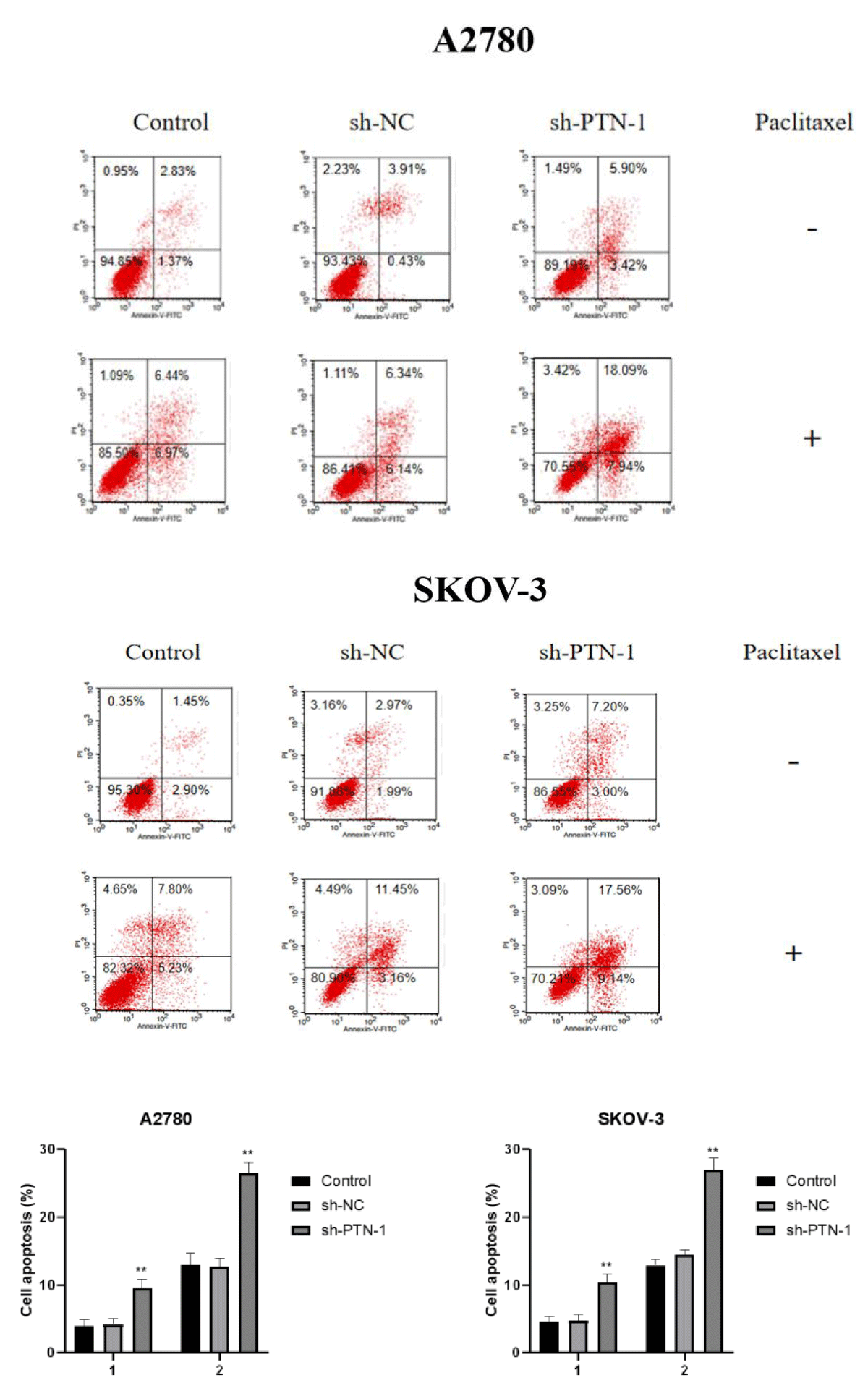
In order to verify the effect of PTN silencing on paclitaxel-induced apoptosis of the two cell lines, paclitaxel with IC50 concentration was added to the control group, the transfected sh-NC group, and the sh-PTN-1 group, and apoptosis was observed after 24 h (Figure 6).
Figure 6: Adding paclitaxel of IC50 concentration to the Control group, sh-NC transfection group and sh-PTN-1 group, and observing apoptosis after 24 h.
It can be concluded from the figure that there is no obvious difference in the total apoptosis rate between the sh-NC group and the control group, but the total apoptosis rate of the sh-PTN-1 group is significantly higher than that of the sh-NC group and the control group, which indicates that A2780 and SKOV-3 cells with silenced PTN expression are more sensitive to paclitaxel.
The expression of cleaved PARP in paclitaxel-treated PTN silenced A2780 and SKOV-3 cells
Paclitaxel of a certain concentration was added to the two cell lines with silenced PTN expression, and the expression of cleaved PARP was detected by Western blot after 48 h of drug action (Figure 7).
Figure 7: 1 means no dosing and 2 means dosing. Compared with the control, the significant difference is expressed by *p < 0.05, **p < 0.01.
It can be seen from the figure that the expression of Cleared PARP in the sh-PTN-1 group is significantly higher than that in the Control group, which indicates that the degree of apoptosis induced by paclitaxel in two cell lines with PTN expression silencing is deepened.
The incidence rate of ovarian cancer is second only to cervical cancer and endometrial cancer among gynecological cancers, but the mortality rate is higher than the sum of the two. Ovarian cancer has the highest mortality rate among all gynecological cancers. In recent years, the incidence rate of ovarian cancer has been rising every year with younger patients than before. According to the clinicopathological and molecular genetic characteristics, the main histological types of ovarian cancer are divided into two subtypes: type I ovarian cancer and type II ovarian cancer [16]. Due to the lack of effective early diagnosis methods, most patients are already in the middle and late stages when diagnosed [17]. At present, the treatment of ovarian cancer is mainly surgery and chemotherapy [18,19]. Chemotherapy is the main treatment for advanced ovarian cancer. However, chemotherapy resistance is one of the main causes of recurrence and death in ovarian cancer patients. On the one hand, chemotherapy drugs try to kill cancer cells through various mechanisms, and on the other hand, chemotherapy drugs activate the self-protection mechanism of cancer cells, so as to resist the effects of drugs. Therefore, the beginning of tumor chemotherapy also means the occurrence of chemotherapy resistance [20]. To clarify the self-defense mechanism of cells stimulated by chemotherapy drugs and explore effective target proteins are effective ways to overcome chemotherapy resistance Paclitaxel is a first-line drug for the treatment of ovarian cancer [21]. At the initial stage of paclitaxel treatment, the effect is remarkable. But with the increase in the number of paclitaxel chemotherapy, cancer cells often develop resistance to chemotherapy drugs, which greatly threatens the life and health of patients [22]. PTN is encoded by the PTN gene, and its size is about 116 kb, which is located on chromosome 7 of the human genome (with 7q33) [23]. PTN is an important driver of tumor angiogenesis, which can promote the proliferation, metastasis, and invasion of tumor cells, and play a key role in the proliferation and metastasis of cancer cells. PTN is believed to play a role in the tumorigenesis of several cancers, including glioblastoma, melanoma, and pancreatic cancer [24-27]. Studies have shown that the pathogenesis of ovarian cancer may be related to PTN and its receptors. Therefore, our research on the sensitivity of PTN to paclitaxel in ovarian cancer cells is of great significance for the treatment of ovarian cancer. In order to further study whether PTN has drug resistance to ovarian cancer cells, in this experiment, we found that PTN protein can promote the proliferation of ovarian cancer cells. And for the ovarian cancer cells administered with paclitaxel, the cell survival rate was enhanced after adding PTN protein. We added different concentrations of PTN when paclitaxel stimulated ovarian cancer cells to cause apoptosis. We found that PTN can reduce the apoptosis rate of ovarian cancer, so PTN can reduce the sensitivity of ovarian cancer cells to paclitaxel. On this basis, we transfected sh-NC, sh-PTN-1, and sh-PTN-2 plasmids into A2780 and SKOV-3 cell lines. There was no significant difference in PTN mRNA and protein expression in the sh-NC group, but PTN mRNA and protein expression in the sh-PTN-1 and sh-PTN-2 groups were significantly decreased. It shows that both sh-PTN plasmids can interfere with the expression of PTN. In the MTT test, the cell survival rate of the sh-PTN-1 group was lower than that of the control group and sh-NC group. In the apoptosis assay, the cell apoptosis rate was higher than that of the control group and sh-NC group. By detecting the expression of cleaved PARP. The results show that the expression of cleaved PARP in the sh-PTN-1 group of the two cell lines is higher than that in the control group, which indicates that PTN has a certain anti-apoptotic effect. PTN enhances the resistance of ovarian cancer cells to paclitaxel, which has been verified in different cell lines of ovarian cancer. The study of this topic can provide a reference for studying the mechanism of drug resistance and treatment of ovarian cancer.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020 Jan;70(1):7-30. doi: 10.3322/caac.21590. Epub 2020 Jan 8. PMID: 31912902.
- Cao W, Chen HD, Yu YW. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chinese Medical Journal. 2021;134(7):783-791.
- Yu J, Hu X, Chen X, Zhou Q, Jiang Q, Shi Z, Zhu H. CNOT7 modulates biological functions of ovarian cancer cells via AKT signaling pathway. Life Sci. 2021 Mar 1;268:118996. doi: 10.1016/j.lfs.2020.118996. Epub 2021 Jan 4. PMID: 33412213.
- Deuel TF, Zhang N, Yeh HJ, Silos-Santiago I, Wang ZY. Pleiotrophin: a cytokine with diverse functions and a novel signaling pathway. Arch Biochem Biophys. 2002 Jan 15;397(2):162-71. doi: 10.1006/abbi.2001.2705. PMID: 11795867.
- Hirofumi J, Yukio A. Midkine: A Novel Prognostic Biomarker for Cancer. Cancers. 2010; 2(2):624-641.
- Li W, Cai JH, Zhang J, Tang YX, Wan L. Effects of cyclooxygenase inhibitors in combination with taxol on expression of cyclin D1 and Ki-67 in a xenograft model of ovarian carcinoma. Int J Mol Sci. 2012;13(8):9741-9753. doi: 10.3390/ijms13089741. Epub 2012 Aug 3. PMID: 22949827; PMCID: PMC3431825.
- Michelotti GA, Tucker A, Swiderska-Syn M, Machado MV, Choi SS, Kruger L, Soderblom E, Thompson JW, Mayer-Salman M, Himburg HA, Moylan CA, Guy CD, Garman KS, Premont RT, Chute JP, Diehl AM. Pleiotrophin regulates the ductular reaction by controlling the migration of cells in liver progenitor niches. Gut. 2016 Apr;65(4):683-92. doi: 10.1136/gutjnl-2014-308176. Epub 2015 Jan 16. PMID: 25596181; PMCID: PMC4504836.
- Meng K, Rodriguez-Peña A, Dimitrov T, Chen W, Yamin M, Noda M, Deuel TF. Pleiotrophin signals increased tyrosine phosphorylation of beta beta-catenin through inactivation of the intrinsic catalytic activity of the receptor-type protein tyrosine phosphatase beta/zeta. Proc Natl Acad Sci U S A. 2000 Mar 14;97(6):2603-8. doi: 10.1073/pnas.020487997. PMID: 10706604; PMCID: PMC15975.
- Liu Y, LiuX, Wang H. Agrimonolide inhibits cancer progression and induces ferroptosis and apoptosis by targeting SCD1 in ovarian cancer cells. Phytomedicine. Phytomedicine.2022; 101.
- Wang Y, Niu XL, Qu Y, Wu J, Zhu YQ, Sun WJ, Li LZ. Autocrine production of interleukin-6 confers cisplatin and paclitaxel resistance in ovarian cancer cells. Cancer Lett. 2010 Sep 1;295(1):110-23. doi: 10.1016/j.canlet.2010.02.019. Epub 2010 Mar 16. PMID: 20236757.
- Zhang J, Wang L, Jiang J. Elevation of microRNA-512-5p inhibits MUC1 to reduce radioresistance in cervical cancer. Cell Cycle. 2020 Mar;19(6):652-665.
- Su C, Liu S, Ma X. The effect and mechanism of erianin on the reversal of oxaliplatin resistance in human colon cancer cells. Cell Biology International. 2021, 45(12): 2420-2428.
- Ruan Z, Yang X, Cheng W. OCT4 accelerates tumorigenesis through activating JAK/STAT signaling in ovarian cancer side population cells. Cancer Manag Res. 2018 Dec 28;11:389-399. doi: 10.2147/CMAR.S180418. PMID: 30643464; PMCID: PMC6314052.
- Zhao Z, Xu Y, Lu J. High expression of HO-1 predicts poor prognosis of ovarian cancer patients and promotes proliferation and aggressiveness of ovarian cancer cells. Clin Transl Oncol. 2018 Apr;20(4):491-499.
- Meng C, Liu K, Cai X, Chen Y. Mechanism of miR-455-3 in suppressing epithelial-mesenchymal transition and angiogenesis of non-small cell lung cancer cells. Cell Stress Chaperones. 2021 Mar;27(2):107-117. doi: 10.1007/s12192-022-01254-4. Epub 2022 Jan 22. Erratum in: Cell Stress Chaperones. 2022 Feb 16;: PMID: 35064898; PMCID: PMC8943084.
- Ricciardelli C, Oehler MK. Diverse molecular pathways in ovarian cancer and their clinical significance. Maturitas. 2009 Mar 20;62(3):270-5. doi: 10.1016/j.maturitas.2009.01.001. Epub 2009 Feb 3. PMID: 19193504.
- McCluggage WG. Morphological subtypes of ovarian carcinoma: a review with emphasis on new developments and pathogenesis. Pathology. 2011 Aug;43(5):420-32. doi: 10.1097/PAT.0b013e328348a6e7. PMID: 21716157.
- Ozols RF. Systemic therapy for ovarian cancer: current status and new treatments. Semin Oncol. 2006 Apr;33(2 Suppl 6):S3-11. doi: 10.1053/j.seminoncol.2006.03.011. PMID: 16716797.
- Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010 Sep;177(3):1053-64. doi: 10.2353/ajpath.2010.100105. Epub 2010 Jul 22. PMID: 20651229; PMCID: PMC2928939.
- Szenajch J, Szabelska-Beręsewicz A, Świercz A, Zyprych-Walczak J, Siatkowski I, Góralski M, Synowiec A, Handschuh L. Transcriptome Remodeling in Gradual Development of Inverse Resistance between Paclitaxel and Cisplatin in Ovarian Cancer Cells. Int J Mol Sci. 2020 Dec 3;21(23):9218. doi: 10.3390/ijms21239218. PMID: 33287223; PMCID: PMC7730278.
- Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA; Gynecologic Oncology Group. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006 Jan 5;354(1):34-43. doi: 10.1056/NEJMoa052985. PMID: 16394300.
- Zhang N, Dai L, Qi Y, Di W, Xia P. Combination of FTY720 with cisplatin exhibits antagonistic effects in ovarian cancer cells: role of autophagy. Int J Oncol. 2013 Jun;42(6):2053-9. doi: 10.3892/ijo.2013.1906. Epub 2013 Apr 17. PMID: 23592281.
- Wang X. Pleiotrophin: Activity and mechanism. Adv Clin Chem. 2020;98:51-89. doi: 10.1016/bs.acc.2020.02.003. Epub 2020 Mar 12. PMID: 32564788; PMCID: PMC7672882.
- Grzelinski M, Steinberg F, Martens T, Czubayko F, Lamszus K, Aigner A. Enhanced antitumorigenic effects in glioblastoma on double targeting of pleiotrophin and its receptor ALK. Neoplasia. 2009 Feb;11(2):145-56. doi: 10.1593/neo.81040. PMID: 19177199; PMCID: PMC2631139.
- Sethi G, Kwon Y, Burkhalter RJ, Pathak HB, Madan R, McHugh S, Atay S, Murthy S, Tawfik OW, Godwin AK. PTN signaling: Components and mechanistic insights in human ovarian cancer. Mol Carcinog. 2015 Dec;54(12):1772-85. doi: 10.1002/mc.22249. Epub 2014 Nov 21. PMID: 25418856; PMCID: PMC4456343.
- Czubayko F, Schulte AM, Berchem GJ, Wellstein A. Melanoma angiogenesis and metastasis modulated by ribozyme targeting of the secreted growth factor pleiotrophin. Proc Natl Acad Sci U S A. 1996 Dec 10;93(25):14753-8. doi: 10.1073/pnas.93.25.14753. PMID: 8962127; PMCID: PMC26208.
- Weber D, Klomp HJ, Czubayko F, Wellstein A, Juhl H. Pleiotrophin can be rate-limiting for pancreatic cancer cell growth. Cancer Res. 2000 Sep 15;60(18):5284-8. PMID: 11016659.