More Information
Submitted: June 10, 2023 | Approved: June 23, 2023 | Published: June 24, 2023
How to cite this article: Amin SA, Alam M, Wang B, Zhen W, Lin C, et al. The Impacts of Angiotensin Receptor Blockers (ARBs) or Angiotensin-converting Enzyme Inhibitors (ACEIs) on Patients with Stereotactic Body Radiation Therapy (SBRT) for Early-Stage NSCLC. J Radiol Oncol. 2023; 7: 033-041.
DOI: 10.29328/journal.jro.1001050
Copyright License: © 2023 Amin SA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The Impacts of Angiotensin Receptor Blockers (ARBs) or Angiotensin-Converting Enzyme Inhibitors (ACEIs) on Patients with Stereotactic Body Radiation Therapy (SBRT) for Early-Stage NSCLC
Saber A Amin1, Morshed Alam2, Bangchen Wang2, Weining Zhen1, Chi Lin1, Apar Kishor Ganti3,4, Vinicius Ernani3, Alissa Marr3, Tony JC Wang5, Simon K Cheng5, Michael Baine1 and Chi Zhang1*
1Department of Radiation Oncology, Fred & Pamela Buffett Cancer Center, University of Nebraska Medical Center, Omaha, NE, USA
2College of Medicine, University of Nebraska Medical Center, Omaha, NE, USA
3Division of Oncology-Hematology, University of Nebraska Medical Center, Fred and Pamela Buffett Cancer Center, Omaha, NE, USA
4Department of Internal Medicine, VA Nebraska Western Iowa Health Care System, Omaha, NE, USA
5Department of Radiation Oncology, Columbia University Irving Medical Center, New York, NY, USA
*Address for Correspondence: Chi Zhang, MD, PhD, Department of Radiation Oncology, Fred & Pamela Buffett Cancer Center, The University of Nebraska Medical Center, Omaha, NE 68198-7521, USA, Email: [email protected]
Purpose: Stereotactic body radiation therapy (SBRT) has emerged as an alternative to surgery for patients with inoperable early-stage non-small cell lung cancer (NSCLC). The majority of inoperable NSCLC patients are elderly and frequently have comorbidities including cardiovascular diseases for which they frequently receive angiotensin receptor blockers (ARBs) or angiotensin-converting enzyme inhibitors (ACEIs). The interactions of these medications with SBRT are not clear. The objective of the current study is to investigate the interaction of ARBs and ACEIs with SBRT for the outcomes of early-stage NSCLC.
Methods and Materials: A retrospective chart review of patients treated with SBRT for Stage I and II NSCLC (AJCC 7th edition) at a single institution between 2006 and 2017 was conducted. Information on the use of ARBs, ACEIs, demographics, and tumor-related factors was collected. Kaplan-Meier and Cox proportional hazard analyses were performed to assess the impact of ARBs and ACEIs combined with SBRT respectively on the treatment outcomes of these patients.
Results: In total, 116 patients were included in the study, among whom 38/116 (32.76%) received ACEIs, and 20/116 (17.24%) received ARBs. In the multivariable analysis, the use of ARBs, but not ACEIs, with SBRT, was significantly associated with the increased risk of dissemination (Hazard Ratio (HR): 2.97; CI: 1.40-6.27; p < 0.004) compared to SBRT without ARBs. The tumor size of > = 3 cm was associated with significantly decreased time to local failure and OS compared to tumor size <3cm.
Conclusion: In the current retrospective study, the use of ARBs, in combination with SBRT, was associated with a significantly increased risk of disease dissemination in early-stage NSCLC compared to SBRT alone. The findings warrant further investigations on the concurrent use of ARBs, ACEIs, and other medicines used for chronic diseases with SBRT for early-stage NSCLC.
Lung cancer patients often present with comorbidities, and many early-stage NSCLC patients with significant comorbidities are not candidates for surgical resection [1]. More than 25% of early-stage NSCLC patients are deemed medically inoperable due to comorbidities including reduced pulmonary functions [2-4]. Stereotactic body radiation therapy (SBRT) has been established as a standard treatment option for these patients with a high local control rate of 90% and a 5-year survival rate of 30% - 50% in early-stage NSCLC [5-7]. Recent data also suggest that, due to long-term efficacy and low toxicity rates, SBRT could be considered as an alternative to surgery after showing comparable outcomes in borderline or even operable early-stage NSCLC [8-12].
A majority of NSCLC patients present with other chronic diseases such as hypertension and heart failure and may take angiotensin I-converting enzyme inhibitors (ACEIs) or angiotensin II type-1 receptor blockers (ARBs) for these diseases [1]. Angiotensin is a critical mediator in the renin-angiotensin system (RAS) pathway and plays a pivotal role not only in maintaining cardiovascular homeostasis and fluid and electrolyte balance but also in tumor progression [13]. The inhibition of RAS suppresses tumor growth, metastases, and angiogenesis which may serve as the mechanism of ACEIs/ARBs’ effects on tumor growth and progress [14,15].
Indeed, studies have indicated that ACEIs and ARBs may reduce metastases and improve survival in certain cancers [2,12,13,16-20]. A meta-analysis reported that the use of (ACEIs) or (ARBs) resulted in significant improvement in disease-free survival (DFS) (HR: 0.60, p = 0.007) and overall survival (OS) in cancer patients (HR: 0.75, p = 0.04) [17]. The analysis by cancer site showed improved DFS in urinary tract cancers (HR: 0.62; CI: 0.44-0.87; p = 0.006), colorectal cancer (HR: 0.22; 95% CI 0.08–0.65; p =0.007), pancreatic cancer (HR: 0.58; 95% CI 0.34–0.95; p = 0.032) and prostate cancer (HR 0.14; 95% CI 0.05–0.36; p < 0.001) but not in breast cancer or hepatocellular carcinoma [17]. No conclusion was reported about lung cancer patients. Of note, pooled data only showed OS benefit for ACEI or ARB users in high-stage dominant tumor studies but not in low-stage dominant studies.
Other studies showed that the use of ACEIs or ARBs also improves DFS and OS in lung cancer [2,12,13,18-20]. Patients with stage III and IV NSCLC who received ACEIs or ARBs had 3.1-3.6 months longer median OS and 2.1 months longer DFS than non-users [13,16,18]. A 3.2-month median longer DFS and nine months longer OS was also reported in ACEIs or ARBs recipients taking tyrosine kinase inhibitors (TKIs) [12,16,19]. A recent meta-analysis investigating the impact of renin-angiotensin system blockers on lung cancer prognosis reported improved OS (HR, 0.86). The subgroup analysis also indicated better OS in NSCLC (HR, 0.78) and advanced-stage patients (HR, 0.77) [20]. Although after separating ACEIs from the ARBs usage groups, neither of the group respectively showed statistically significant OS benefit with HR for the ACEIs group being 0.83 but the ARBs group being close to 1 (0.95). The difference in the HRs indicates the OS benefit may be mainly contributed to the use of ACEI. Interestingly, recent studies have indicated that the use of ACEIs is also associated with decreased toxicity and pneumonitis after radiation therapy in NSCLC [21,22].
Given the overall benefit of SBRT and the antiproliferative effect of ACEIs and/or ARBs, the interaction of these two treatments may have a synergetic or additive effect on the prognosis of NSCLC. To our knowledge, there has been no study that has looked at the interaction of ACEIs/ARBs particularly separately, or any other antihypertensive drugs with SBRT in early-stage NSCLC and investigated its impact on OS, local control, and disease dissemination. The objective of the present analysis is to investigate the interaction of SBRT with ACEIs or ARBs respectively on the local control, disease dissemination (nodal and/or distal failure), and overall survival of NSCLC patients.
Patient selection
We performed a retrospective chart review on individual patients treated with SBRT for AJCC 7th edition Stage I and II NSCLC at the University of Nebraska Medical Center between 2006 and 2017. Inclusion criteria for this study were: patients had no prior lung cancer or previous cancer in remission for > 5 years; primary stage I/II lung cancer; non-small cell lung cancer only (small cell carcinomas were deleted); no systemic therapy before or after SBRT unless it was used as salvage for disease progression and the patient should have had at least one follow-up imaging after SBRT. Simultaneously diagnosed NSCLCs were included if both lesions were staged as I and/or II separately and both were treated with SBRT. Baseline demographic and treatment characteristics were recorded. The use of ACEIs and ARBs was recorded as documented in the hospital’s electronic medical record systems as well as the date of initial prescription, if available. We compared the patients who received ACEIs with SBRT to those who received SBRT without ACEIs (including ARBs). We also compared the cohort on ARBs combined with SBRT to those not using ARBs. All of these patients received these medicines prior to and concurrently with SBRT. Disease dissemination was defined as patients with regional nodal and/or distant metastasis including intrathoracic metastasis in a different lobe or extrathoracic distal metastasis. The study was approved by the University of Nebraska Institutional Review Board (IRB). Inform consent was obtained from patients during the treatment visit.
Statistical analysis
Variables included for the analyses were age, gender, tumor size, histological types of lung cancer, and use of ACEI or ARB. OS was calculated from the date of disease diagnosis to the patient’s death from any cause. Patients alive at the last follow-up or lost to follow-up were censored. We calculated the local failure time from the date of diagnosis to the date of local failure as the first progression and the dissemination failure time from the date of diagnosis to the date of dissemination as the first progression. We used the Kaplan-Meier (KM) method to generate survival curves and analyze the differences between them with the log-rank test.
The Cox proportional hazard regression analysis was conducted to estimate hazard ratios and their associated 95% confidence intervals. The Multivariate Cox regression model included the variables of use of ACEIs or ARBs, age at diagnosis, sex, race, tumor size, and histological type of lung cancer. Proportional hazard assumption assumptions were tested using the log-log test and no violation of the assumptions was noticed. We used the p value of .05 to define statistical significance. Data analyses were performed with SAS 9.4 (SAS Enterprise, Cary, NC).
Characteristics of the study cohort
In total, charts of 182 patients who received SBRT treatment for early-stage NSCLC at our institute from 2006 to 2017 were reviewed. Of these, 116 patients met the inclusion criteria and were included in data analyses. The median age at diagnosis was 75.7 years, ranging from 52-94 years, with lower and upper quartiles of (70-79) years. Among all patients, 61/116 (52.6%) were male, 55/116 (47.4%) were female, 102/115 (88.7%) were White, 13/115 (11.3%) non-white, 105/116 (90.5%) had tumor size <3cm, 11/116 (9.5%) had tumor size > 3 cm, 52/116 (44.8%) were adenocarcinoma, 43/116 (37.1%) were squamous cell carcinoma and the rest (18.1%) were other histological subtypes of lung cancer. Overall, 38/116 (32.7%) patients received ACEIs, 20/116 (17.24%) received ARBs, 78/116 (67.2%) did not receive ACEIs and 96/116 (82.76%) did not receive ARBs. None of the patients received both drugs. All of these patients received these medicines prior to and concurrently with SBRT. The characteristics of the study population are provided in Table 1.
| Table 1: Demographic characteristics of the patient population. | ||
| Characteristics | N (%) | |
| Age of diagnosis (continuous) Median 75.70 (52-94) | 116 | |
| Gender | Male | 61 (52.59%) |
| Female | 55 (47.41%) | |
| Race | White | 102 (88.70%) |
| Other | 13 (11.30%) | |
| Tumor Size | < 3 cm | 105 (91.52%) |
| > = 3 cm | 11 (9.48% | |
| Histology | Squamous Cell Carcinoma | 43 (37.07%) |
| Non-squamous Cell | 73 (62.93%) | |
| ARBs | Yes | 20 (17.24%) |
| No | 96 (82.76%) | |
| ACEIs | Yes | 38 (32.76%) |
| No | 78 (67.24%) | |
| Status | Death | 58 (56.86%) |
| Alive | 44 (43.14%) | |
| Local Failure | Yes | 8 (6.90%) |
| No | 108 (93.10%) | |
| Dissemination Failure | Yes | 34 (30.63%) |
| No | 77 (69.37%) | |
| Missing | 5 | |
| Nodal Failure | Yes | 8 (7.02%) |
| No | 106 (92.98%) | |
| Missing | 2 | |
| Overall Survival (median) | 37.90 (CI: 30.00-44.50) | |
| Dissemination-free time (median) | 55.20 (CI: 36.20-not reached) | |
Treatment outcomes of the study cohort
The median follow-up time was 30.3 months for the study cohort of patients. Fourteen patients were excluded from the overall survival analysis due to simultaneous diagnosis of two types of NSCLC. Among the remaining 102 patients, 58 (56.86%) died during the follow-up period. Among the entire cohort, there were 8 (6.90%) local failures, 8 (7.02%) nodal failures, and 23 (19.8%) distant failures. Among the eight patients who developed local recurrence, the median time to local recurrence was 16.9 months. The median time to dissemination was 19.8 months in the 34 patients (30.63%) who developed dissemination disease.
Univariate analyses of ARBs and ACEIs plus SBRT
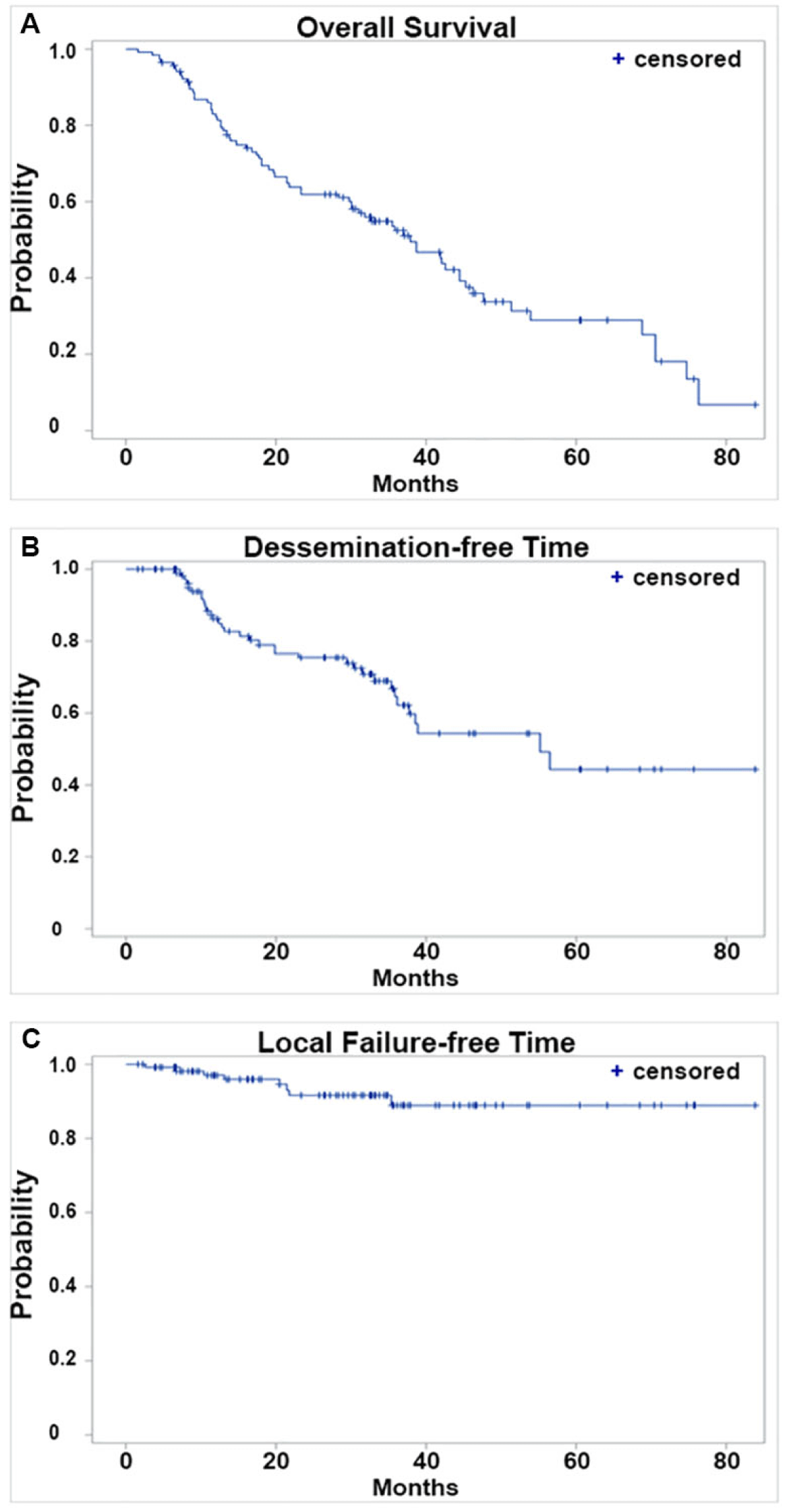
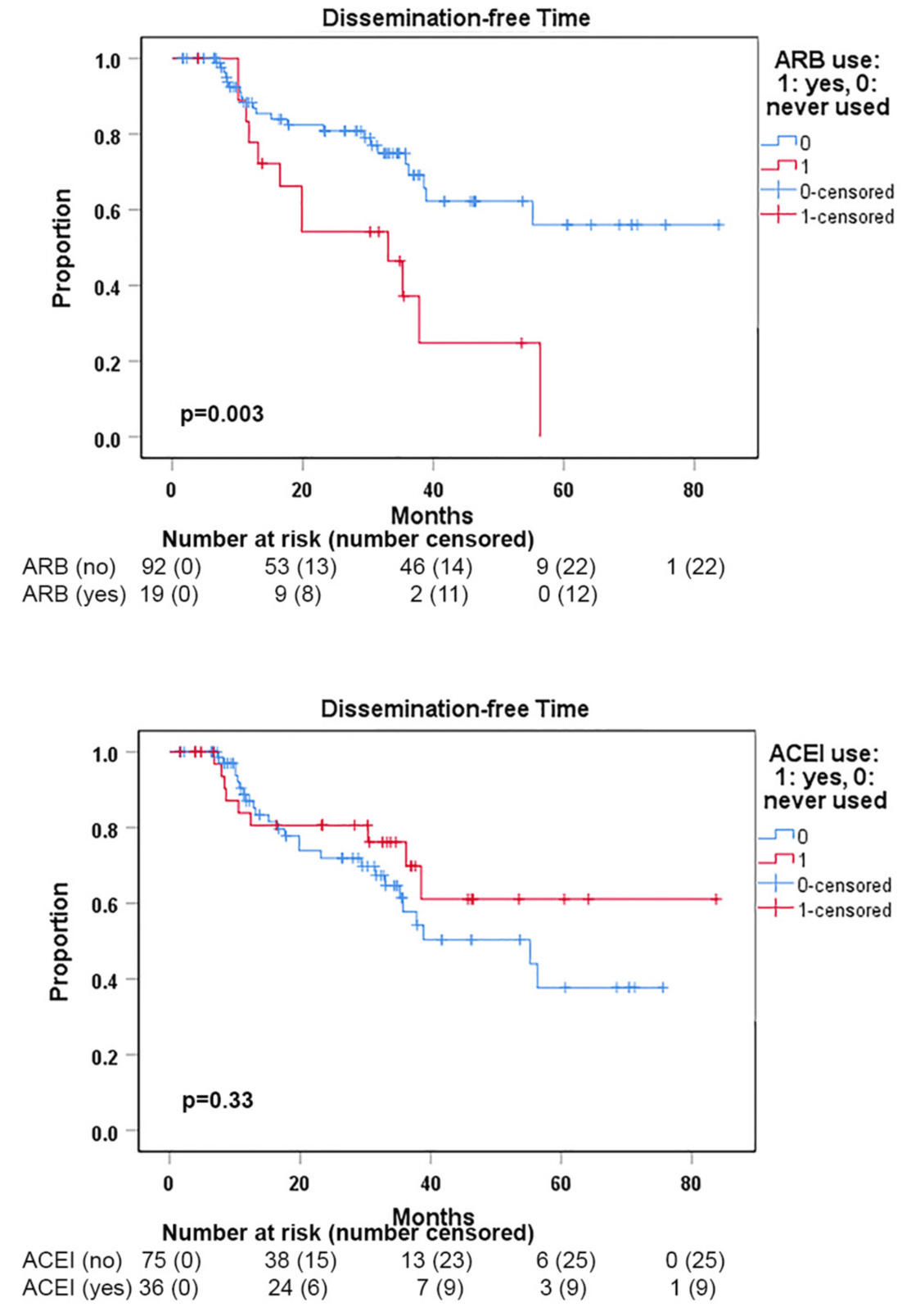
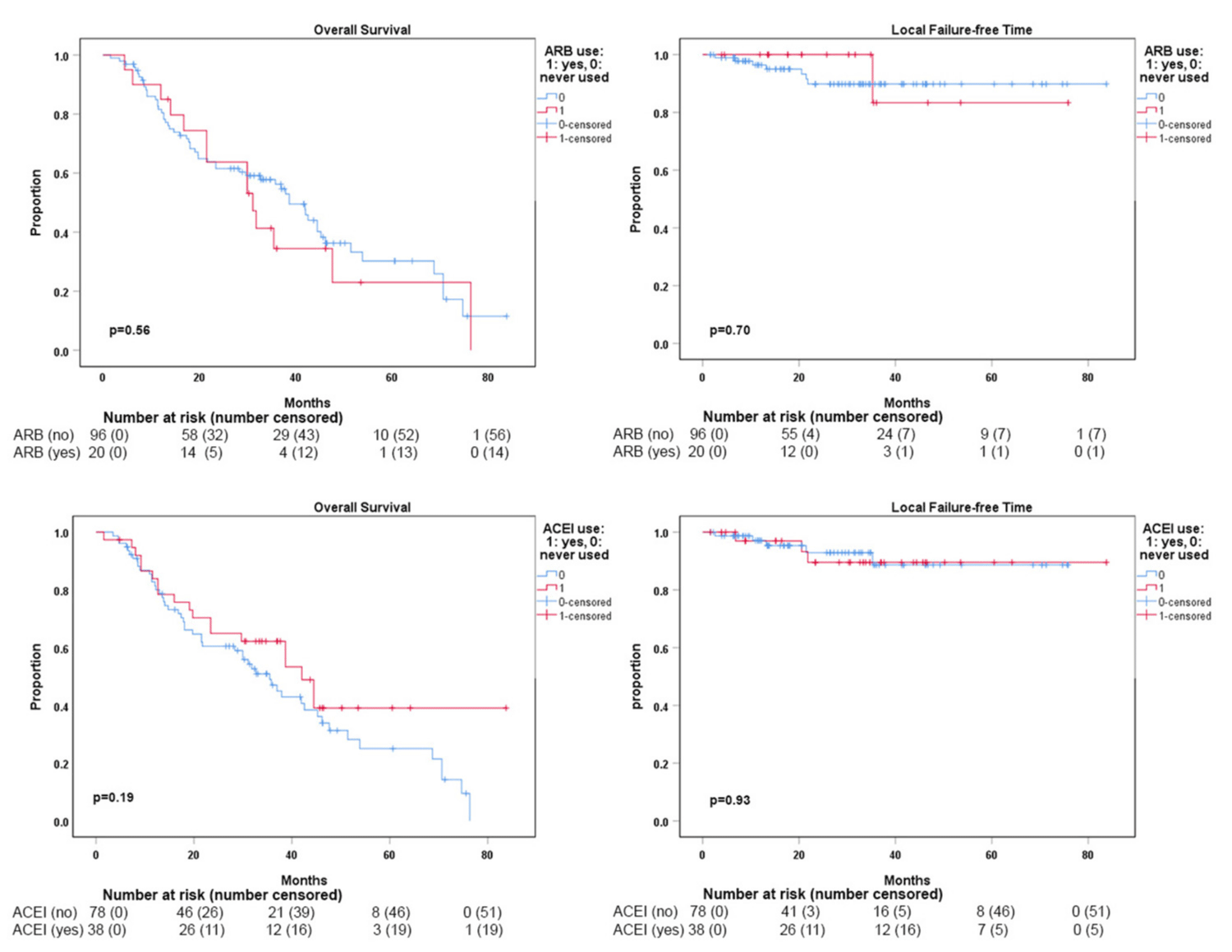
The median OS for the entire cohort was 37.9 months (Figure 1A). The median dissemination-free time (DFT) for the entire cohort was 55.2 months (Figure 1B). The median local failure-free time (LFFT) was not reached (Figure 1C). Kaplan-Meier analysis showed that DFT was significantly shorter in patients who received ARBs with SBRT compared to those who did not use ARBs (p = 0.003) (Figure 2A). SBRT with or without ACEI use did not show a significant difference in DFTs (Figure 2B). The use of ARBs with SBRT was not associated with OS (Figure 3a) and LFF time (Figure 3B). The use of ACEIs with SBRT was also not associated with OS (Figure 3C) and LFFT (Figure 3D). In the univariate Cox proportional analysis for LFFT, only tumor size > = 3 cm was associated with significantly decreased LFFT (HR: 5.80, CI: 1.12-30.02; p = 0.036) compared to tumor size < 3 cm. In the univariate Cox analysis for DFT, only the use of ARBs with SBRT was associated with significantly reduced DFT (HR: 2.76, CI: 1.36-5.61; p = 0.005) compared to SBRT without the use of ARBs. Tumor size >= 3 cm was associated with decreased OS (HR: 2.75, CI:1.29-5.87) compared to tumor size < 3 cm. The univariate and multivariate Cox proportional hazard analysis results are provided in Tables 2,3.
Figure 1: Kaplan-Meier analyses of treatment outcomes of the entire study cohort. A. Overall survival; B. Dissemination-free time; and C. Local failure-free time of all patients included in our study.
Figure 2: Kaplan-Meier analyses of dissemination-free time (DFT) from disease diagnosis to first dissemination showed significantly worse outcomes in ARB users when compared with non-ARB users (p = 0.03) but no significant difference between ACEI users vs. non-ACEI users (p = 0.33).
Figure 3: Overall survival (A) and local failure-free time (B) comparisons between ARB users vs. non-ARB users. Overall survival (C) and local failure-free time (D) comparisons between ACEI users vs. non-ACEI users. None of the comparisons showed statistically significant differences.
| Table 2: (A) Univariate and multivariate analysis for dissemination-free time (DFT) for the use of ARBs plus SBRT vs. SBRT without ARBs (A), and for the use of ACEIs plus SBRT vs. SBRT without ACEIs (B). | |||||
| Variables for DFT | Univariate analysis | P | Multivariate analysis | P | |
| Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | ||||
| Age of diagnosis (continuous) | 1.017 (0.971-1.065) | 0.476 | 1.028 (0.978-1.081) | 0.276 | |
| ARBs | Yes | 2.760 (1.358-5.612) | 0.005 | 2.965 (1.402-6.268) | 0.004 |
| No | Ref | Ref | |||
| Sex | Male | 1.400 (0.710-2.760) | 0.332 | 1.521 (0.763-3.031) | 0.233 |
| Female | Ref | Ref | |||
| Race | White | 0.00 (0.00-…) | 0.987 | 0.000 (0.000-…) | 0.988 |
| Other | Ref | Ref | |||
| Tumor Size | < 3 cm | Ref | Ref | ||
| > = 3 cm | 2.010 (0.600-6.727) | 0.258 | 5.162 (0.734-36.283) | 0.099 | |
| Histology | Squamous Cell | 0.835 (0.407-1.714) | 0.624 | 0.667 (0.319-1.392) | 0.281 |
| Other Types | Ref | Ref | |||
| Table 2B | |||||
| Age of diagnosis (continuous) | 1.017 (0.971-1.065) | 0.476 | 1.020 (0.968-1.074) | 0.456 | |
| ACEIs | Yes | 0.688 (0.321-1.476) | 0.337 | 0.752 (0.341-1.658) | 0.480 |
| No | Ref | Ref | |||
| Sex | Male | 1.400 (0.710-2.760) | 0.332 | 1.526 (0.768-3.033) | 0.228 |
| Female | Ref | Ref | |||
| Race | White | 0.00 (0.00-…) | 0.987 | 0.000 (0.000-…) | 0.987 |
| Other | Ref | Ref | |||
| Tumor Size | < 3 cm | Ref | Ref | ||
| > = 3 cm | 2.010 (0.600-6.727) | 0.258 | 1.935 (0.559-6.703) | 0.298 | |
| Histology | Squamous Cell | 0.835 (0.407-1.714) | 0.624 | 0.698 (0.337-1.447) | 0.334 |
| Other Types | Ref | Ref | |||
| Table 3: (A) Univariate and multivariate analysis for local failure-free time (LFFT) for the use of ACEIs plus SBRT vs. SBRT without ACEIs (A). Univariate and multivariate analysis for OS for the use of ARBs plus SBRT vs. SBRT without ARBs (B), and for the use of ACEIs plus SBRT vs. SBRT without ACEIs (C). | |||||
| Variables for LFFT | Univariate analysis | p | Multivariate analysis | p | |
| Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | ||||
| Age of diagnosis (continuous) | 1.000 (0.913-1.096) | 1.00 | 0.971 (0.876-1.077) | 0.582 | |
| ACEIs | Yes | 1.133 (0.270-4.760) | 0.865 | 1.958 (0.395-9.708) | 0.411 |
| No | Ref | Ref | |||
| Sex | Male | 3.260 (0.657-16.181) | 0.148 | 3.731 (0.682-20.417) | 0.129 |
| Female | Ref | Ref | |||
| Race | White | 0.00 (0.00-…) | 0.994 | 0.000 (0.000-…) | 0.995 |
| Other | Ref | Ref | |||
| Tumor Size | < 3 cm | Ref | Ref | ||
| > = 3 cm | 5.799 (1.120-30.019) | 0.036 | 6.726 (1.086-41.650) | 0.041 | |
| Histology | Squamous Cell | 1.744 (0.435-6.987) | 0.432 | 1.258 (0.312-5.070) | 0.746 |
| Other Types | Ref | Ref | |||
| Table 3B | |||||
| Age of diagnosis (continuous) | 1.011 (0.977-1.046) | 0.530 | 1.010 (0.973-1.049) | 0.607 | |
| ARBs | Yes | 1.130 (0.583-2.191) | 0.717 | 1.235 (0.611-2.496) | 0.557 |
| No | Ref | Ref | |||
| Sex | Male | 1.232 (0.735-2.064) | 0.429 | 1.150 (0.669-1.979) | 0.613 |
| Female | Ref | Ref | |||
| Race | White | 0.575 (0.208-1.592) | 0.287 | 0.584 (0.200-1.702) | 0.325 |
| Other | Ref | Ref | |||
| Tumor Size | < 3 cm | Ref | Ref | ||
| > = 3 cm | 2.746 (1.286-5.866) | 0.009 | 2.645 (1.187-5.891) | 0.017 | |
| Histology | Squamous Cell | 1.589 (0.944-2.674) | 0.081 | 1.438 (0.837-2.471) | 0.188 |
| Other Types | Ref | Ref | |||
| Table 3c | |||||
| Age of diagnosis (continuous) | 1.011 (0.977-1.046) | 0.530 | 1.011 (0.974-1.050) | 0.560 | |
| ACEIs | Yes | 0.609 (0.333-1.113) | 0.107 | 0.637 (0.343-1.183) | 0.153 |
| No | Ref | Ref | |||
| Sex | Male | 1.232 (0.735-2.064) | 0.429 | 1.169 (0.686-1.993) | 0.566 |
| Female | Ref | Ref | |||
| Race | White | 0.575 (0.208-1.592) | 0.287 | 0.561 (0.192-1.644) | 0.292 |
| Other | Ref | Ref | |||
| Tumor Size | < 3 cm | Ref | Ref | ||
| > = 3 cm | 2.746 (1.286-5.866) | 0.009 | 2.253 (1.005-5.050) | 0.049 | |
| Histology | Squamous Cell | 1.589 (0.944-2.674) | 0.081 | 1.456 (0.847-2.501) | 0.174 |
| Other Types | Ref | Ref | |||
Multivariate analyses of ARBs and ACEIs with SBRT
In the multivariate analysis (MVA) adjusted for age at diagnosis, gender, tumor size, race, and histology, the use of ARBs with SBRT was associated with a significantly increased risk of dissemination (HR: 2.97; CI: 1.40-6.27; p < 0.004) compared to SBRT without ARBs (Table 2A). The use of ACEIs with SBRT, however, was not associated with the risk of dissemination (HR: 0.75, CI: 0.34-1.66; p = 0.480) (Table 2B). The use of ARBs with SBRT was not significantly associated with the risk of local failure in MVA (HR: 0.64, CI: 0.07-6.04, p = 0.694) (data not shown). The use of ACEIs with SBRT was not significantly associated with LFFT (HR: 1.92, CI: 0.29-9.52, p = 0.426) (Table 3A). Interestingly in the MVA of LFT for the use of ACEIs with SBRT, tumor size >= 3cm was associated with the increased risk of local failure (HR: 6.73, CI: 1.09-41.65; p = 0.041) compared to tumor size < 3 cm (Table 3A). The use of ARBs with SBRT was not associated with significantly improved OS (HR: 1.24, CI: 0.61-2.50; p = 0.557). Tumor size of > = 3 cm was associated with significantly decreased OS (HR: 2.65, CI: 1.19-5.89; p = 0.017) compared to tumor size < 3 cm in the MVA of investigating the impact of ARBs with SBRT for OS (Table 3B). The use of ACEIs was not associated with OS (HR: 0.64, CI: 0.34-1.18; p = 0.153) compared to SBRT without ACEIs. In the MVA of the ACEIs with the SBRT model, tumor size of > = 3 cm again was associated with significantly decreased OS (HR: 2.25, CI: 1.01-5.05; p = 0.048) compared to tumor size < 3 cm (Table 3C).
The primary purpose of this study was to examine whether exposure to ARBs or ACEIs prior to and during SBRT is associated with improved survival and tumor progression in early-stage NSCLC. In this single-institution retrospective study, we found that the use of ARBs with SBRT was associated with a significantly increased risk of tumor dissemination (nodal and/or distal failure) in multivariable analysis but not local control or overall survival. No negative impact was noticed in patients who received ACEIs with SBRT.
Importantly, our data contrast with previous works which suggested a survival benefit with the use of ARBs in advanced stages of NSCLC [2,12,13,18-20], which could be partly explained by the fact that those studies mostly combined the use of ACEIs and ARBs for data analyses which may have diluted the adverse effects of ARBs specifically. The benefit shown in advanced-stage NSCLCs could be due to the additional use of systemic therapy such as chemotherapy mitigating the potentially harmful component of the effects of ARBs. Current standard SBRT dose regimens, when biologically equivalent dose (BED) is above 100 Gy (ablative dose), provide sufficient local control without the need for systemic therapy or concurrent use of sensitizing therapies [11] which could explain our finding that concurrent use of ACEIs or ARBs did not further improve local control in early stage NSCLC. However, for advanced-stage NSCLC, due to the large thoracic volumes of RT, the BED of thoracic RT usually stops at 60-80 Gy. Thus, an ablative dose of RT to the primary tumor or nodal metastasis cannot be reached even with concurrent chemotherapy in which case patients may benefit from concurrent use of ACEIs/ARBs potentially explaining the observed benefit of concurrent use of ARBs in advanced stages of NSCLC [2,12,13,18-20]. The possible underlying reason for our findings of increased dissemination in ARB users in early-stage NSCLC may be attributable to the systemic interaction of ARBs which may promote immune evasion of cancer cells and tumor metastasis.
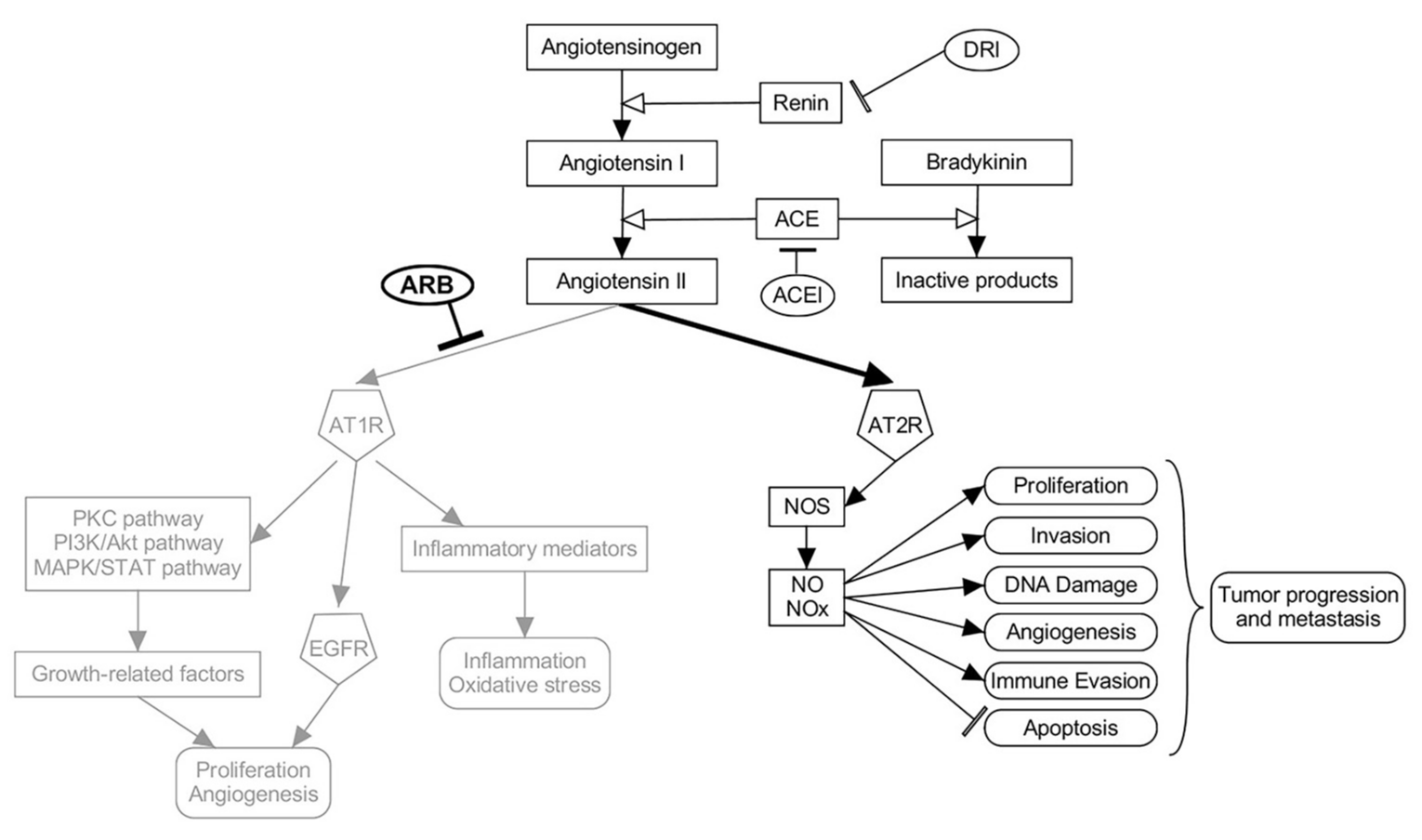
The ARBs have an essential protective role in the cardiovascular system through angiotensin receptor 1(AT1) via the release of protein kinase C and nitric oxide (NO) [23]. The ARBs inhibit the binding of angiotensin II to AT1 receptors [24]. The ARBs do not have a direct effect on bradykinin, but they may still increase the release of nitric oxide and prevent its degradation [24]. Blocking AT1 receptor by ARBs also can potentially shift and thus increase the activity of angiotensin II on the AT2 receptor, which in turn leads to an increase in the nitric oxide synthase and NO level, which is different from the mechanism of ACEIs that block both receptor pathways by decreasing angiotensin II levels overall [25]. An increase in NO and nitric radicals is associated with tumor angiogenesis, invasion, and metastasis [26]. Studies have shown that NO limits T cell proliferation and affects the antitumor host response causing tumor growth and spread [27]. Tumor cells, such as myeloid-derived suppressor cells (MDSC), use the secretion of NO as a mechanism for immune evasion [28]. In lung cancer, NO has a significant effect on P53, glycolysis, and cell growth pathways, which may create a tumor microenvironment that promotes tumorigenesis and metastases [29]. Nitric oxide synthase in lymphatic endothelial cells may mediate lymphangiogenesis, lymphatic hypertrophy, dilation, and thus metastasis [30], a possible biological explanation for the findings of our study in which we noted a significantly increased risk of dissemination in ARBs recipients. This effect and pathways are highlighted in Figure 4. It remains to be answered whether ARB uses prior to SBRT renders patients at higher risk of harboring occult regional nodal or distal micrometastasis, or whether concurrent use of ARBs with SBRT promotes a higher chance of disease dissemination. It is also interesting to see that, although ARB use is significantly associated with increased risks of disease dissemination, OS is not compromised, likely due to effective salvage options or a high proportion of patients dying of non-cancer-related causes, particularly in this cohort being a non-surgical candidate.
Figure 4: Renin-Angiotensin model explaining potential mechanisms of ARB promoting early-stage NSCLC dissemination. The overactivation of the AT2R pathway by Angiotensin II after blocking AT1R by ARBs may promote tumor metastasis and immune evasion.
The association of larger tumor size with decreased LFFT and OS indicates that local failure is most likely a predicting factor for poor survival. Our database, however, due to limited sample size and few incidences of local failure, is not able to answer the question of whether concurrent use of ACEIs/ARBs with SBRT can further improve local control on large-sized tumors.
The major strength of our proposed study is that this is the first study that has investigated the different impacts of ARBs and ACEIs on the prognosis of early-stage NSCLC patients treated with SBRT without systemic therapy. The present study also has several limitations that must be considered while interpreting the data. It is a single-institution study, and may not be representative of a broader population. Our study also had a small sample size which may be responsible for some insignificant associations and sample bias. Some other limitations of the proposed research are the retrospective nature of the study, the heterogeneous population, and the lack of information on when most drugs were initiated or stopped, so we were unable to assess the effects of the duration of use of ARBs prior to SBRT.
In conclusion, our data indicate that, in contrast to studies in advanced stage NSCLC which showed survival benefit of ACEIs/ARBs, patients on ARBs when receiving SBRT for early stage NSCLC are associated with higher risks of disease dissemination without local control benefit. ACEIs do not carry the same risks. It is critical to understand the impact of ARBs/ACEIs on the prognosis of NSCLC when used with SBRT as SBRT has become a widely adopted treatment in the early stages of this disease. The findings of our study need to be further validated in more extensive multi-institutional or prospective studies but current results support adding adjuvant systemic therapy such as immunotherapy after SBRT for early-stage NSCLC which is currently under clinical investigation.
Future perspective
Future studies should use data from multiple institutions with a large sample size. In the current study, we combined nodal failure with distant failure due to not having enough events. Future studies should also focus on the use of ARBs and ACEIs concurrently with SBRT and after SBRT. It is very critical to determine when to use these drugs with SBRT in early-stage NSCLC.
Summary points
- The use of ARBs with SBRT was associated with a higher hazard of cancer dissemination
- In the OS model of the ARBs and SBRT cohort, tumor size > = 3cm was associated with decreased OS compared to tumor size < 3 cm
- In the OS model of the ACEIs cohort, tumor size > = 3 cm was associated with decreased OS compared to tumor size < 3 cm
- In the multivariable analysis for local failure in the ACEIs and SBRT cohort, tumor size>=3cm was associated with a higher hazard of local failure compared to tumor size < 3 cm.
- Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, Jemal A, Cho H, Anderson RN, Kohler BA, Eheman CR, Ward EM. Annual Report to the Nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014 May 1;120(9):1290-314. doi: 10.1002/cncr.28509. Epub 2013 Dec 16. PMID: 24343171; PMCID: PMC3999205.
- Febbo JA, Gaddikeri RS, Shah PN. Stereotactic Body Radiation Therapy for Early-Stage Non-Small Cell Lung Cancer: A Primer for Radiologists. Radiographics. 2018 Sep-Oct;38(5):1312-1336. doi: 10.1148/rg.2018170155. Epub 2018 Aug 3. PMID: 30074857.
- Sebastian NT, Xu-Welliver M, Williams TM. Stereotactic body radiation therapy (SBRT) for early stage non-small cell lung cancer (NSCLC): contemporary insights and advances. J Thorac Dis. 2018 Aug;10(Suppl 21):S2451-S2464. doi: 10.21037/jtd.2018.04.52. PMID: 30206491; PMCID: PMC6123192.
- Kastelijn EA, El Sharouni SY, Hofman FN, Van Putte BP, Monninkhof EM, Van Vulpen M, Schramel FM. Clinical Outcomes in Early-stage NSCLC Treated with Stereotactic Body Radiotherapy Versus Surgical Resection. Anticancer Res. 2015 Oct;35(10):5607-14. PMID: 26408733.
- Wisnivesky JP, Bonomi M, Henschke C, Iannuzzi M, McGinn T. Radiation therapy for the treatment of unresected stage I-II non-small cell lung cancer. Chest. 2005 Sep;128(3):1461-7. doi: 10.1378/chest.128.3.1461. PMID: 16162744.
- Cardia J, Calçada C, Pereira H. Treatment of lung cancer in the elderly: Influence of comorbidity on toxicity and survival. Rep Pract Oncol Radiother. 2011 Feb 23;16(2):45-8. doi: 10.1016/j.rpor.2011.01.001. PMID: 24376955; PMCID: PMC3863214.
- Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman BJ, Senan S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol. 2010 Dec 10;28(35):5153-9. doi: 10.1200/JCO.2010.30.0731. Epub 2010 Nov 1. PMID: 21041709.
- Timmerman RD, Paulus R, Pass HI, Gore EM, Edelman MJ, Galvin J, Straube WL, Nedzi LA, McGarry RC, Robinson CG, Schiff PB, Chang G, Loo BW Jr, Bradley JD, Choy H. Stereotactic Body Radiation Therapy for Operable Early-Stage Lung Cancer: Findings From the NRG Oncology RTOG 0618 Trial. JAMA Oncol. 2018 Sep 1;4(9):1263-1266. doi: 10.1001/jamaoncol.2018.1251. PMID: 29852037; PMCID: PMC6117102.
- Zheng X, Schipper M, Kidwell K, Lin J, Reddy R, Ren Y, Chang A, Lv F, Orringer M, Spring Kong FM. Survival outcome after stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: a meta-analysis. Int J Radiat Oncol Biol Phys. 2014 Nov 1;90(3):603-11. doi: 10.1016/j.ijrobp.2014.05.055. Epub 2014 Jul 19. PMID: 25052562.
- Shibamoto Y, Hashizume C, Baba F, Ayakawa S, Miyakawa A, Murai T, Takaoka T, Hattori Y, Asai R. Stereotactic body radiotherapy using a radiobiology-based regimen for stage I non-small-cell lung cancer: five-year mature results. J Thorac Oncol. 2015 Jun;10(6):960-4. doi: 10.1097/JTO.0000000000000525. PMID: 26001145.
- Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, Karasawa K, Hayakawa K, Niibe Y, Takai Y, Kimura T, Takeda A, Ouchi A, Hareyama M, Kokubo M, Kozuka T, Arimoto T, Hara R, Itami J, Araki T. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys. 2011 Dec 1;81(5):1352-8. doi: 10.1016/j.ijrobp.2009.07.1751. Epub 2010 Jul 16. PMID: 20638194.
- Aydiner A, Ciftci R, Sen F. Renin-Angiotensin system blockers may prolong survival of metastatic non-small cell lung cancer patients receiving erlotinib. Medicine (Baltimore). 2015 Jun;94(22):e887. doi: 10.1097/MD.0000000000000887. PMID: 26039117; PMCID: PMC4616356.
- Wilop S, von Hobe S, Crysandt M, Esser A, Osieka R, Jost E. Impact of angiotensin I converting enzyme inhibitors and angiotensin II type 1 receptor blockers on survival in patients with advanced non-small-cell lung cancer undergoing first-line platinum-based chemotherapy. J Cancer Res Clin Oncol. 2009 Oct;135(10):1429-35. doi: 10.1007/s00432-009-0587-3. Epub 2009 Apr 28. PMID: 19399518.
- George AJ, Thomas WG, Hannan RD. The renin-angiotensin system and cancer: old dog, new tricks. Nat Rev Cancer. 2010 Nov;10(11):745-59. doi: 10.1038/nrc2945. Epub 2010 Oct 22. PMID: 20966920.
- Pinter M, Jain RK. Targeting the renin-angiotensin system to improve cancer treatment: Implications for immunotherapy. Sci Transl Med. 2017 Oct 4;9(410):eaan5616. doi: 10.1126/scitranslmed.aan5616. PMID: 28978752; PMCID: PMC5928511.
- Miao L, Chen W, Zhou L, Wan H, Gao B, Feng Y. Impact of Angiotensin I-converting Enzyme Inhibitors and Angiotensin II Type-1 Receptor Blockers on Survival of Patients with NSCLC. Sci Rep. 2016 Feb 17;6:21359. doi: 10.1038/srep21359. PMID: 26883083; PMCID: PMC4756359.
- Song T, Choi CH, Kim MK, Kim ML, Yun BS, Seong SJ. The effect of angiotensin system inhibitors (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers) on cancer recurrence and survival: a meta-analysis. Eur J Cancer Prev. 2017 Jan;26(1):78-85. doi: 10.1097/CEJ.0000000000000269. PMID: 27158979.
- Menter AR, Carroll NM, Sakoda LC, Delate T, Hornbrook MC, Jain RK, Kushi LH, Quinn VP, Ritzwoller DP. Effect of Angiotensin System Inhibitors on Survival in Patients Receiving Chemotherapy for Advanced Non-Small-Cell Lung Cancer. Clin Lung Cancer. 2017 Mar;18(2):189-197.e3. doi: 10.1016/j.cllc.2016.07.008. Epub 2016 Aug 20. PMID: 27637408; PMCID: PMC5424707.
- Linhai Zhu, Jian Xiao Qu, Zhaofei Pang. The impact of hypertension and renin-angiotensin system blockers on outcomes of lung cancer patients: A population-based retrospective cohort study. Int J Clin Exp Pathol. 2017; 10(4): 4818-4826.
- Wang N, Liu J, Wang W. The impact of renin-angiotensin system blockers on lung cancers prognosis: A prisma-compliant systematic review and meta-analysis. Allied J Med Res. 2017; 1(1): 28-34.
- Small W Jr, James JL, Moore TD, Fintel DJ, Lutz ST, Movsas B, Suntharalingam M, Garces YI, Ivker R, Moulder J, Pugh S, Berk LB. Utility of the ACE Inhibitor Captopril in Mitigating Radiation-associated Pulmonary Toxicity in Lung Cancer: Results From NRG Oncology RTOG 0123. Am J Clin Oncol. 2018 Apr;41(4):396-401. doi: 10.1097/COC.0000000000000289. PMID: 27100959; PMCID: PMC5073047.
- Alite F, Balasubramanian N, Adams W, Surucu M, Mescioglu I, Harkenrider MM. Decreased Risk of Radiation Pneumonitis With Coincident Concurrent Use of Angiotensin-converting Enzyme Inhibitors in Patients Receiving Lung Stereotactic Body Radiation Therapy. Am J Clin Oncol. 2018 Jun;41(6):576-580. doi: 10.1097/COC.0000000000000324. PMID: 27560156.
- Jugdutt BI. Valsartan in the treatment of heart attack survivors. Vasc Health Risk Manag. 2006;2(2):125-38. doi: 10.2147/vhrm.2006.2.2.125. PMID: 17319456; PMCID: PMC1993995.
- Munger MA. Use of Angiotensin receptor blockers in cardiovascular protection: current evidence and future directions. P T. 2011 Jan;36(1):22-40. PMID: 21386934; PMCID: PMC3046622.
- Dézsi CA. The Different Therapeutic Choices with ARBs. Which One to Give? When? Why? Am J Cardiovasc Drugs. 2016 Aug;16(4):255-266. doi: 10.1007/s40256-016-0165-4. PMID: 26940560; PMCID: PMC4947116.
- Ying L, Hofseth LJ. An emerging role for endothelial nitric oxide synthase in chronic inflammation and cancer. Cancer Res. 2007 Feb 15;67(4):1407-10. doi: 10.1158/0008-5472.CAN-06-2149. PMID: 17308075.
- Choudhari SK, Chaudhary M, Bagde S, Gadbail AR, Joshi V. Nitric oxide and cancer: a review. World J Surg Oncol. 2013 May 30;11:118. doi: 10.1186/1477-7819-11-118. PMID: 23718886; PMCID: PMC3669621.
- Markowitz J, Wang J, Vangundy Z, You J, Yildiz V, Yu L, Foote IP, Branson OE, Stiff AR, Brooks TR, Biesiadecki B, Olencki T, Tridandapani S, Freitas MA, Papenfuss T, Phelps MA, Carson WE. Nitric oxide mediated inhibition of antigen presentation from DCs to CD4+ T cells in cancer and measurement of STAT1 nitration. Sci Rep. 2017 Nov 13;7(1):15424. doi: 10.1038/s41598-017-14970-0. Erratum in: Sci Rep. 2018 Mar 6;8(1):4203. PMID: 29133913; PMCID: PMC5684213.
- Masri FA, Comhair SA, Koeck T, Xu W, Janocha A, Ghosh S, Dweik RA, Golish J, Kinter M, Stuehr DJ, Erzurum SC, Aulak KS. Abnormalities in nitric oxide and its derivatives in lung cancer. Am J Respir Crit Care Med. 2005 Sep 1;172(5):597-605. doi: 10.1164/rccm.200411-1523OC. Epub 2005 Jun 9. PMID: 15947282; PMCID: PMC2718532.
- Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat Rev Cancer. 2006 Jul;6(7):521-34. doi: 10.1038/nrc1910. PMID: 16794635.