More Information
Submitted: August 14, 2023 | Approved: August 23, 2023 | Published: August 24, 2023
How to cite this article: Moore-Palhares D, Saifuddin M, Ho L, Lu L, Dasgupta A, et al. A Novel Strategy to Improve Radiotherapy Effectiveness: First-in-Human MR-guided Focused Ultrasound-Stimulated Microbubbles (MRgFUS+MB) Radiation Enhancement Treatment. J Radiol Oncol. 2023; 7: 047-051.
DOI: 10.29328/journal.jro.1001052
Copyright License: © 2023 Moore-Palhares D, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Microbubbles; Radiation-sensitizing agents; Magnetic resonance imaging; Focused ultrasound; Ultrasonography; Head and neck neoplasms
Abbreviated title: MB: Microbubbles; FUS: Focused Ultrasound; ASMase: Acid Sphingomyelinase; SBRT: Stereotactic Body Radiotherapy; MR: Magnetic Resonance; MRgFUS+MB: Magnetic Resonance-Guided Focused Ultrasound-Stimulated MB
A Novel Strategy to Improve Radiotherapy Effectiveness: First-in-Human MR-guided Focused Ultrasound-Stimulated Microbubbles (MRgFUS+MB) Radiation Enhancement Treatment
Daniel Moore-Palhares1-3, Murtuza Saifuddin3, Ling Ho1, Lin Lu1, Archya Dasgupta1-3, Martin Smoragiewicz4, Irene Karam1,2, Andrew Bayley1,2, Arjun Sahgal1,2, Ian Poon1,2 and Gregory J Czarnota1-3,5*
1Department of Radiation Oncology, Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada
2Department of Radiation Oncology, University of Toronto, Toronto, Ontario, Canada
3Physical Sciences, Sunnybrook Research Institute, Toronto, Ontario, Canada
4Department of Medical Oncology, University of Toronto, Toronto, Ontario, Canada
5Department of Biophysics, University of Toronto, Toronto, Ontario, Canada
*Address for Correspondence: Gregory J Czarnota, PhD, MD, FRCPC, Department of Radiation Oncology, Sunnybrook Health Sciences Centre, 2075 Bayview Avenue, T2, Toronto, Ontario M4N3M5, Canada, Email: [email protected]
Background and aim: Preclinical in vitro and in vivo experiments suggest that radiation-induced tumour cell death can be enhanced 10- to 40-fold when combined with focused-ultrasound (FUS)-stimulated microbubbles (MB). The acoustic exposure of MB in the tumour volume causes vasculature perturbation, activation of the acid sphingomyelinase (ASMase) ceramide pathway, and resultant endothelial cell apoptosis. When the tumour is subsequently treated with radiation, there is increased endothelial cell death and anoxic tumour killing. Here we describe a first-in-human experience treating patients with magnetic resonance (MR)-guided FUS-stimulated MB (MRgFUS+MB) radiation enhancement.
Case presentation: A head and neck cancer patient with recurrent disease underwent radiotherapy for 5 separate sites of locoregional disease followed by systemic therapy. The first consisted of a course of 45 Gy in 5 fractions alone, the second of 30 Gy in 5 fractions with hyperthermia, and the three others of 20-30 Gy in 5 fractions along with MRgFUS+MB treatment. The treatment methodology used an MR-coupled FUS-device operating at 500 KHz and 540 kPa peak negative pressure with an insonification time of 750 ms spread over 5 minutes to stimulate intravenously administered MB within tumour target. All sites treated with stimulated MB had a complete radiological response, and subsequently, the patient’s other cutaneous metastatic disease disappeared. The patient has been under surveillance for over two years without active treatment or disease progression.
Discussion: MRgFUS+MB was well-tolerated with no reported treatment-related adverse events, which can be attributed to the capability of FUS to selectively stimulate MB within the tumour volume while sparing the surrounding normal tissue. Sustained local control at all target sites aligns with earlier preclinical findings suggesting the radiation enhancement potential of FUS+MB.
Conclusion: MRgFUS+MB represents a novel and promising therapy for enhancing radiation efficacy and improving therapeutic index with potential improvements in disease control.
High-dose radiation (i.e., 70 Gy in 35 fractions) with concurrent platinum-based chemotherapy is the standard treatment for unresectable locally advanced head and neck cancer [1,2]. Chemotherapy acts as a radiosensitizer, enhancing the radiation effect and improving outcomes [1,2]. However, despite intensive treatment, locoregional relapse is still the most common pattern of treatment failure [1,3]. Moreover, concurrent chemotherapy comes at the cost of increased toxicity, partly due to its inability to selectively sensitize the tumour. Therefore, there is an unmet need for developing new radiosensitizers that could selectively improve radiotherapy efficacy without increasing treatment toxicity.
Microbubbles (MB) are gas microspheres (< 10 μm) stabilized by a thin shell of lipids, routinely used as intravascular ultrasound contrast agents. The acoustic exposure of MB using focused ultrasound (FUS) causes bubble cavitation with resultant shockwaves capable of inducing transient perturbation of vasculature. This, in turn, activates the acid sphingomyelinase (ASMase) ceramide pathway that results in subsequent endothelial cell apoptosis [4-6]. Evidence suggests that this mechanism is essential for the ablative effect of stereotactic body radiotherapy (SBRT) when fractions > 8 - 10 Gy are delivered [5,7]. It was demonstrated, however, that ASMase ceramide-mediated endothelial apoptosis can be induced with doses as low as 2 Gy in a single fraction when radiotherapy is combined with stimulated MB. This synergism increases tumour endothelial cell death, leading to microvascular destruction, impairment of tumour blood supply, and subsequent anoxic tumour killing [4,6]. This principle has been proven by extensive preclinical work in different cancer models [4,5,6,8-10]. For example, using a prostate cancer model, Czarnota, et al. [8] demonstrated a mean tumour cell death of only 4% (± 2%) for a 2 Gy fraction alone, but at least 10-fold more significant cell kill was observed when combining ultrasound-stimulated MB with a single 2 Gy fraction (44% ± 13%) or a single 8 Gy fraction (70% ± 8%) [8].
Given the compelling preclinical data results, we have started an ongoing phase I clinical trial of magnetic resonance (MR)-guided FUS-stimulated MB (MRgFUS+MB) enhancement treatment in patients with unresectable or inoperable primary head and neck malignancies (clinicaltrials.gov identifier: NCT04431648). Here we describe the first patient treated with this innovative therapy.
A 48-year-old male with a squamous cell carcinoma of the left buccal mucosa underwent tumour resection with positive margins (pT3N0) followed by concurrent chemoradiotherapy. Radiotherapy consisted of a dose of 56 Gy to the ipsilateral nodal levels with a simultaneous integrated boost of 66 Gy to the surgical bed using an intensity-modulated radiotherapy technique with 33 fractions.
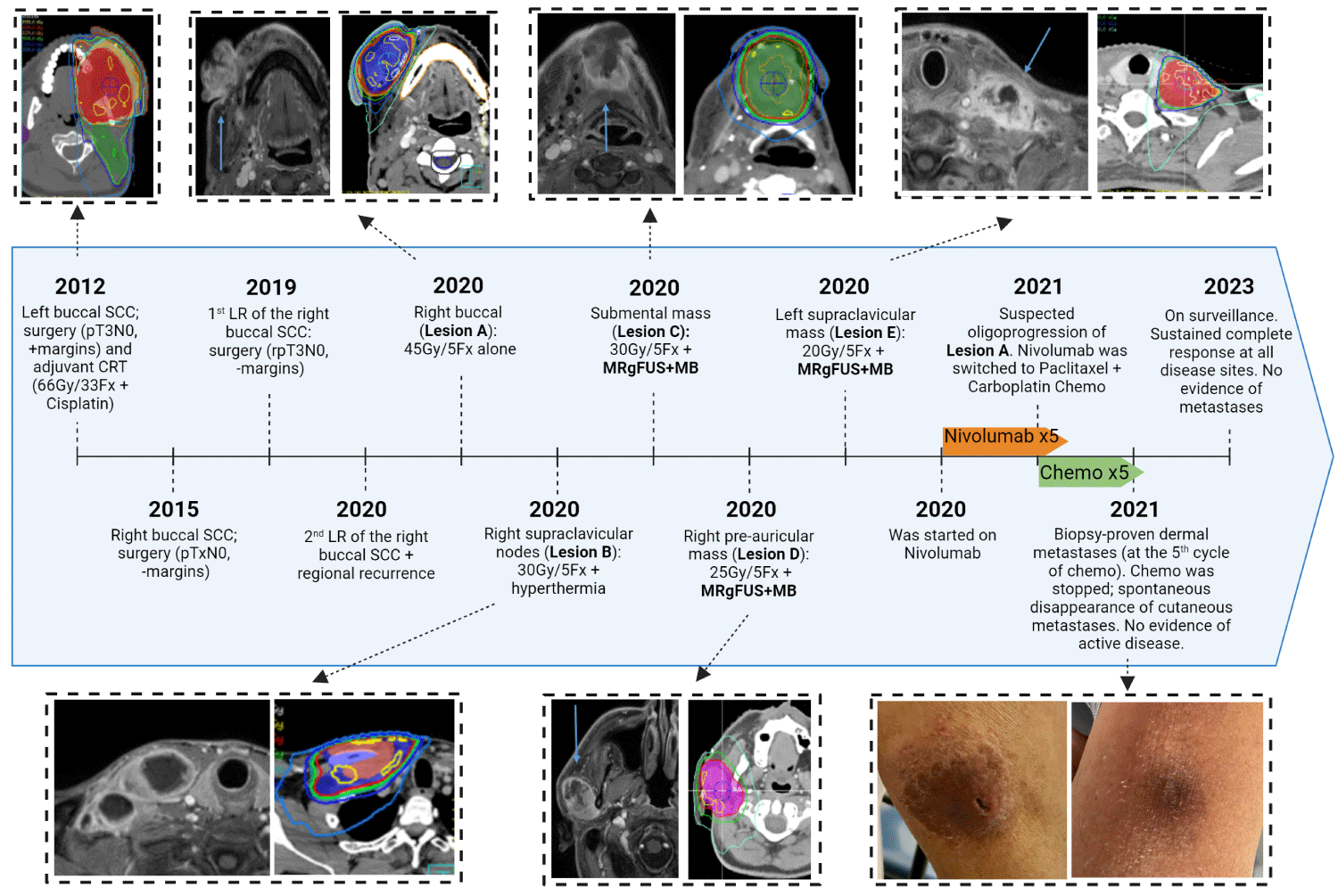
A second primary of the right buccal mucosa developed, which was completely resected. Four years later, he developed the first local recurrence of this tumour, which was resected again with negative margins (4.4 cm squamous cell carcinoma), and no adjuvant treatment was delivered. Eight months later, he presented another local recurrence in the right buccal mucosa (termed “lesion A”), and this was associated with enlarged right supraclavicular nodes (“lesion B”).
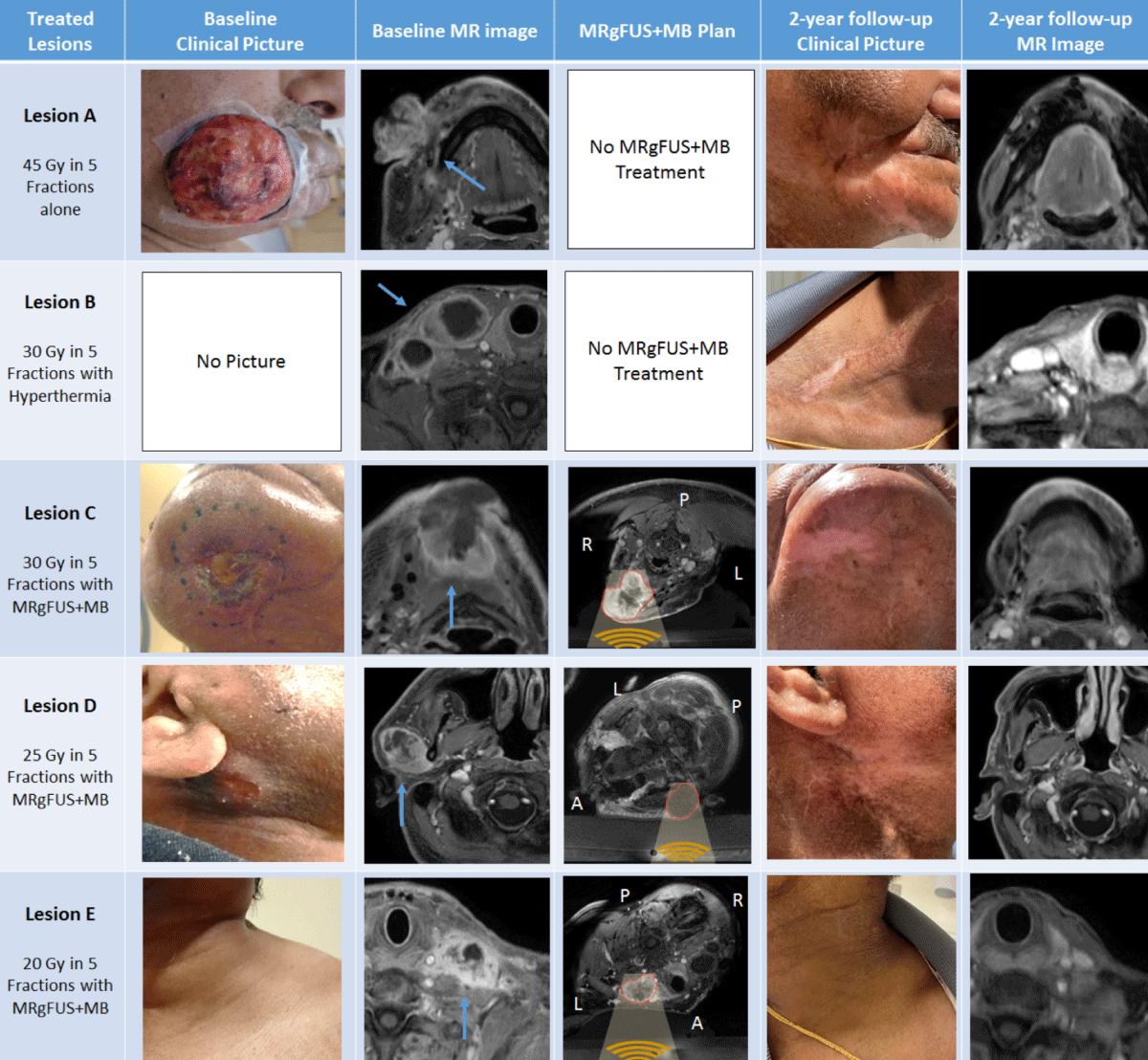
Lesion A was treated with SBRT alone with a dose of 45 Gy in 5 fractions, and lesion B using a dose of 30 Gy in 5 fractions along with MR-guided FUS hyperthermia (as part of a separate clinical trial). Three months later, upon developing a new submental mass (“lesion C”), he was enrolled in the stimulated MB phase 1 trial, and lesion C was treated with a radiation dose of 30 Gy in 5 fractions along with MRgFUS+MB treatment. He then presented enlargement of a pre-auricular adenopathy (“lesion D”) and a left supraclavicular conglomerate (“lesion E”), which were treated with radiation doses of 25 Gy in 5 fractions and 20 Gy in 5 fractions, respectively, along with concurrent MRgFUS+MB treatment.
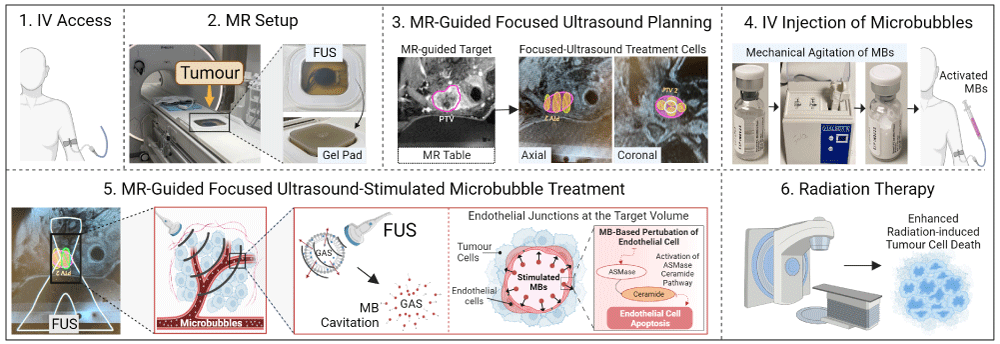
The MRgFUS+MB treatments were delivered immediately before radiation fractions 1 and 5. A FUS system (Profound Medical/Philips Sonalleve - Profound Medical, Mississauga, Canada/ Philips Healthcare, Best, Netherlands) was integrated with a 3-Tesla MR system platform (Philips Healthcare, Netherlands) and reprogrammed with alterations to frequency and power (from 1.2 MHz - 1.4 MHz and 270 W - 300 W to 0.8 MHz and 7.3 W, respectively) to sonicate tumours at depowered operating conditions that cause no heating nor tissue damage. On the MRgFUS+MB treatment days, the patient was positioned on the MR table with the target in close contact with the in-built FUS transducer (Figure 1). Ultrasound gel was applied for coupling purposes on the top of a gel pad (Aquaflex; Parker, Hannover, Germany), facilitating contact with the ultrasound transducer without air gaps. The treating radiation oncologist used the acquired MR images to define the target volume and place individual ultrasound treatment cells that covered the entire target volume. MB (Lantheus Medical Imaging, USA) were activated by vigorous mechanical agitation for 45 seconds using a Vialmix unit (Lantheus Medical Imaging, USA), then injected intravenously at a dose of 10-30 μL/kg per treatment cell. Subsequently, FUS sonicated the MB within the targeted volume using a peak negative pressure of 570 kPa until the whole volume was treated. The patient was monitored for 30 minutes post-procedure and underwent radiotherapy within one hour of MRgFUS+MB treatment completion.
Figure 1: Methodology of magnetic resonance-guided focused ultrasound-stimulated microbubbles radiation enhancement treatment. 1. On the treatment days, a peripheral intravenous line was inserted to administer contrast media and microbubbles. 2. The patient was positioned on the magnetic resonance table with the target tumour in close contact with the focused ultrasound transducer. Ultrasound gel was applied for coupling purposes on the top of a gel pad, facilitating contact with the ultrasound transducer without air gaps. 3. The treating radiation oncologist used the acquired magnetic resonance images to define the target tumour and place individual ultrasound treatment cells that cover the entire target. 4. The microbubbles were activated by vigorous mechanical agitation for 45 seconds using a Vialmix unit (Lantheus Medical Imaging, USA) and then injected intravenously at a dose of 10-30 μL/kg per each ultrasound treatment cell. 5. Focused ultrasound sonicated the microbubbles within the targeted volume using a peak negative pressure of 570 kPa until the whole volume was treated. The acoustic exposure of microbubbles causes vasculature perturbation, activation of the acid sphingomyelinase (ASMase) ceramide pathway, and resultant endothelial cell apoptosis. 6. The patient was transferred to the linear accelerator to undergo radiotherapy within one hour of completing the magnetic resonance-guided focused ultrasound-stimulated microbubbles treatment. Subsequent radiation treatment leads to increased endothelial cell death, resulting in microvascular destruction, impairment of the tumour blood supply, and enhanced tumour cell death. Abbreviations: IV: Intravenous; FUS: Focused Ultrasound; MR: Magnetic Resonance; MB: Microbubbles; ASMase: Acid Sphingomyelinase.
The five radiotherapy courses were followed by immune checkpoint inhibitors (Nivolumab). At the fifth cycle, Nivolumab was switched to Paclitaxel/Carboplatin chemotherapy due to suspected oligoprogression of lesion A while all other sites had complete radiological response. Lesion A responded to the new regimen, but during the fifth cycle of chemotherapy, the patient developed biopsy-proven widespread cutaneous metastases. Systemic treatment was discontinued, and surprisingly, this was followed by spontaneous resolution of all cutaneous lesions within 1-month after the last chemotherapy. Since then, the patient has been on surveillance for over two years (Figures 2 and 3)
Figure 2: Contrast enhanced CT of the abdomen in the axial (a), coronal (b) and sagittal (c) planes showing a left retroperitoneal suprarenal cyst (star) pushing the left kidney posteroinferiorly, the left adrenal gland (a, b; white arrow) medially, and the stomach superoanteriorly. The cyst is adjacent to the left adrenal gland, pancreas, and stomach. There is no fat tissue between them.
Figure 3: Clinical and radiological response to radiotherapy. Abbreviations: MR: Magnetic Resonance; MRgFUS+MB: Magnetic Resonance-Guided Focused Ultrasound-Stimulated Microbubble Treatment; L: Left; R: Right; P: Posterior; A: Anterior.
with no evidence of active disease. At the last follow-up, he had sustained complete resolution of all disease sites, and the toxicities were within the expected limit (grade 1 xerostomia, grade 2 trismus, grade 2 dysphagia, and skin hypopigmentation). No MRgFUS+MB treatment-related adverse events were reported.
We described the first patient treated with MRgFUS+MB treatment as a novel strategy to enhance radiotherapy effectiveness. This therapy is supported by extensive preclinical research, which showed that FUS+MB synergistically activates cellular pathways that ultimately cause tumour vasculature disrupture and enhance radiation-induced cell death [4,5,6,8-10].
The MRgFUS+MB treatment was well-tolerated, with the expected radiation-induced toxicity and no local or systemic MB-related adverse events. The FUS selectively stimulates MB within the tumour volume, sparing the surrounding normal tissue. This is achievable due to a modern three-dimensional ultrasound technology that permits focused exposure with better than 1 mm accuracy. As a result, any toxicity of stimulated MB enhancement therapy and intended local bioeffects is expected to be restricted to the target tumour. Additionally, the MB utilized in our research (Definity, Lantheus Medical Imaging, USA) has been commercially employed as an ultrasound contrast agent for many years and has exhibited a secure safety profile, with severe adverse events seen in < 0.01% [11,12].
Due to MRgFUS+MB treatment selectivity, one of the main applications is to sensitize the tumour so the radiation dose can be de-escalated when toxicity is a major concern while providing at least the same local control as a higher radiotherapy dose could. Interestingly, this strategy was demonstrated here as the prescribed dose was progressively reduced on subsequent treatments, but no post-MRgFUS+MB treatment recurrence was reported. Moreover, stimulated MB safety and efficacy were further demonstrated as the lesions treated with higher radiation doses (lesions A-B) were internal controls of those treated with MRgFUS+MB (lesions C-E).
We posit that the MRgFUS+MB treatments could have contributed to the priming of the immune system through the exposure of tumour antigens associated with the cell death that rapidly ensues, leading to enhanced tumour ablation. This theory is supported by emerging data suggesting that radiation [13,14] and ultrasound-stimulated MB [15] remodel tumour microenvironment and increase immune cell infiltration, resulting in an enhanced immunotherapeutic effect. It is intriguing, however, that after an excellent response, the patient developed biopsy-proven cutaneous metastases that spontaneously disappeared following chemotherapy interruption. We hypothesize that the stimulated immune system led to the patient’s excellent initial response. However, this was subsequently counterbalanced by chemotherapy-induced immunosuppression, allowing widespread metastatic disease development. Then, when chemotherapy was discontinued, upregulation of a previously stimulated immune system resulted in the disappearance of cutaneous metastases and, ultimately, sustained response.
We reported the successful experience of the first patient treated with MRgFUS+MB radiotherapy enhancement treatment. This represents a novel and promising treatment for enhancing radiation efficacy and improving the therapeutic index with potential improvements in disease control. The safety and effectiveness shown in the presented case need to be confirmed by the ongoing clinical study.
We express our sincere gratitude to the patient involved in the study.
Trial registration: Clinicaltrials.gov, identifier NCT-04431674
Funding
Terry Fox Research Institute with support from the Lotte and John Hecht Memorial Foundation (Grant number 1083).
- Lacas B, Carmel A, Landais C, Wong SJ, Licitra L, Tobias JS, Burtness B, Ghi MG, Cohen EEW, Grau C, Wolf G, Hitt R, Corvò R, Budach V, Kumar S, Laskar SG, Mazeron JJ, Zhong LP, Dobrowsky W, Ghadjar P, Fallai C, Zakotnik B, Sharma A, Bensadoun RJ, Ruo Redda MG, Racadot S, Fountzilas G, Brizel D, Rovea P, Argiris A, Nagy ZT, Lee JW, Fortpied C, Harris J, Bourhis J, Aupérin A, Blanchard P, Pignon JP; MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 107 randomized trials and 19,805 patients, on behalf of MACH-NC Group. Radiother Oncol. 2021 Mar;156:281-293. doi: 10.1016/j.radonc.2021.01.013. Epub 2021 Jan 27. PMID: 33515668; PMCID: PMC8386522.
- Machiels JP, René Leemans C, Golusinski W, Grau C, Licitra L, Gregoire V; EHNS Executive Board. Electronic address: [email protected]; ESMO Guidelines Committee. Electronic address: [email protected]; ESTRO Executive Board. Electronic address: [email protected]. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020 Nov;31(11):1462-1475. doi: 10.1016/j.annonc.2020.07.011. Epub 2020 Oct 23. PMID: 33239190.
- Bollen H, van der Veen J, Laenen A, Nuyts S. Recurrence Patterns After IMRT/VMAT in Head and Neck Cancer. Front Oncol. 2021 Sep 16;11:720052. doi: 10.3389/fonc.2021.720052. PMID: 34604056; PMCID: PMC8483718.
- Czarnota GJ. Ultrasound-stimulated microbubble enhancement of radiation response. Biol Chem. 2015 Jun;396(6-7):645-57. doi: 10.1515/hsz-2014-0297. PMID: 25741736.
- El Kaffas A, Al-Mahrouki A, Hashim A, Law N, Giles A, Czarnota GJ. Role of Acid Sphingomyelinase and Ceramide in Mechano-Acoustic Enhancement of Tumor Radiation Responses. J Natl Cancer Inst. 2018 Sep 1;110(9):1009-1018. doi: 10.1093/jnci/djy011. PMID: 29506145; PMCID: PMC6136928.
- Sharma D, Leong KX, Czarnota GJ. Application of Ultrasound Combined with Microbubbles for Cancer Therapy. Int J Mol Sci. 2022 Apr 15;23(8):4393. doi: 10.3390/ijms23084393. PMID: 35457210; PMCID: PMC9026557.
- Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, Haimovitz-Friedman A, Cordon-Cardo C, Kolesnick R. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001 Jul 13;293(5528):293-7. doi: 10.1126/science.1060191. PMID: 11452123.
- Czarnota GJ, Karshafian R, Burns PN, Wong S, Al Mahrouki A, Lee JW, Caissie A, Tran W, Kim C, Furukawa M, Wong E, Giles A. Tumor radiation response enhancement by acoustical stimulation of the vasculature. Proc Natl Acad Sci U S A. 2012 Jul 24;109(30):E2033-41. doi: 10.1073/pnas.1200053109. Epub 2012 Jul 9. PMID: 22778441; PMCID: PMC3409730.
- Lai P, Tarapacki C, Tran WT, El Kaffas A, Lee J, Hupple C, Iradji S, Giles A, Al-Mahrouki A, Czarnota GJ. Breast tumor response to ultrasound mediated excitation of microbubbles and radiation therapy in vivo. Oncoscience. 2016 Mar 24;3(3-4):98-108. doi: 10.18632/oncoscience.299. Erratum in: Oncoscience. 2017 Jan 30;4(1-2):14. PMID: 27226983; PMCID: PMC4872648.
- Tran WT, Iradji S, Sofroni E, Giles A, Eddy D, Czarnota GJ. Microbubble and ultrasound radioenhancement of bladder cancer. Br J Cancer. 2012 Jul 24;107(3):469-76. doi: 10.1038/bjc.2012.279. Epub 2012 Jul 12. PMID: 22790798; PMCID: PMC3405216.
- Wei K, Mulvagh SL, Carson L, Davidoff R, Gabriel R, Grimm RA, Wilson S, Fane L, Herzog CA, Zoghbi WA, Taylor R, Farrar M, Chaudhry FA, Porter TR, Irani W, Lang RM. The safety of deFinity and Optison for ultrasound image enhancement: a retrospective analysis of 78,383 administered contrast doses. J Am Soc Echocardiogr. 2008 Nov;21(11):1202-6. doi: 10.1016/j.echo.2008.07.019. Epub 2008 Oct 10. PMID: 18848430.
- Weiss RJ, Ahmad M, Villanueva F, Schmitz S, Bhat G, Hibberd MG, Main ML; CaRES Investigators. CaRES (Contrast Echocardiography Registry for Safety Surveillance): a prospective multicenter study to evaluate the safety of the ultrasound contrast agent definity in clinical practice. J Am Soc Echocardiogr. 2012 Jul;25(7):790-5. doi: 10.1016/j.echo.2012.04.002. Epub 2012 May 3. PMID: 22560734.
- Arina A, Gutiontov SI, Weichselbaum RR. Radiotherapy and Immunotherapy for Cancer: From "Systemic" to "Multisite". Clin Cancer Res. 2020 Jun 15;26(12):2777-2782. doi: 10.1158/1078-0432.CCR-19-2034. Epub 2020 Feb 11. PMID: 32047000.
- Wu J, Waxman DJ. Immunogenic chemotherapy: Dose and schedule dependence and combination with immunotherapy. Cancer Lett. 2018 Apr 10;419:210-221. doi: 10.1016/j.canlet.2018.01.050. PMID: 29414305; PMCID: PMC5818299.
- Liu S, Zhang Y, Liu Y, Wang W, Gao S, Yuan W, Sun Z, Liu L, Wang C. Ultrasound-targeted microbubble destruction remodels tumour microenvironment to improve immunotherapeutic effect. Br J Cancer. 2023 Mar;128(5):715-725. doi: 10.1038/s41416-022-02076-y. Epub 2022 Dec 3. PMID: 36463323; PMCID: PMC9977958.