More Information
Submitted: September 21, 2023 | Approved: October 03, 2023 | Published: October 04, 2023
How to cite this article: Alimzhanov NY, Chakeev ISh, Lepshin BN, Kudaibergenova IO, Shaimurzayeva BA, et al. Therapy of Walker Carcinosarcoma with Pectin and Cyclophosphane. J Radiol Oncol. 2023; 7: 066-070.
DOI: 10.29328/journal.jro.1001056
Copyright License: © 2023 Alimzhanov NY, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Nanodimensional pectin; Cyclophosphane; Walker carcinoma
Therapy of Walker Carcinosarcoma with Pectin and Cyclophosphane
NY Alimzhanov, ISh Chakeev, BN Lepshin, IO Kudaibergenova, BA Shaimurzayeva, LV Serikova and Sh Jorobekova*
Institute of Chemistry and Chemical Technology, National Academy of Sciences, Kyrgyzstan, Russia
*Address for Correspondence: Sh Jorobekova, Institute of Chemistry and Chemical Technology, NAS of Kyrgyz Republic, Chui Prospect, 267, 720071, Bishkek, Kyrgyzstan, Russia, Email: [email protected]
Scientific interest in low-molecular-weight pectins is not accidental. Despite the experimental material widely presented in the literature on the pharmacological effects of pectins, the clinical application of the developments has not yet been fully implemented. On the one hand, antitumor potential is registered in polymers with a mass of hundreds of kilodaltons, on the other hand, practically nothing is known about such in pectin derivatives weighing less than 20 kDa. In addition, the issues of assessing the nature of the pharmacological interaction of nanoscale pectin and conventional cytostatics are not covered. The aim of this work is an experimental study of the antitumor potential of low-molecular, low-esterified pectin in combination with a cytostatic agent on a model of Walker’s carcinosarcoma. Pectin therapy of Walker’s transplanted tumor in several series of experiments consistently caused inhibition of its growth from 60% to 80%. The combined use of pectin and cyclophosphane caused inhibition of tumor growth up to 72.4%. The increase in life expectancy in the “pectin + cyclophosphane” group versus the “cyclophosphane” group was 200%. It can be concluded that nanoscale pectin is a promising drug for in-depth study since it meets the criteria of primary screening (increase in animal life expectancy, inhibition of tumor growth, survival without tumor growth).
Pectins, as representatives of polysaccharides, have a wide range of useful pharmacological properties associated with the complex organization of these heteropolysaccharides [1-6]. One of the pharmacological properties of pectins is their high antitumor activity against colon cancer, breast cancer, small cell myeloma, and hemangiosarcoma [7-9]. The detected effectiveness is due to the presence in the pectin molecule of a large amount of β-galactose, which has binding activity against the galectin-3 protein [10,11], which is responsible for the growth and metastasis of tumors. Pectins not only exhibit independent antitumor effects but are also capable of increasing the activity of a number of known anticancer drugs [12]. Thus, a group of oncologists from several Medical Universities in Poland are discussing the prospects of using pectins to increase the selectivity of the action of the well-known antitumor drug irinotecan, widely used in the treatment of colon cancer. It has been shown that the composition and enzymatic activity of the colon microbiota can significantly influence the effectiveness of chemotherapy with this drug. In the intestine, under the action of the bacterial β-glucuronidase enzyme, the medicinal metabolite of irinotecan, its glucuronide (SN-38G), due to which it exhibits antitumor activity, is converted back into its original form (SN-38), which has very high intestinal toxicity. Enzymatically extracted apple pectin very successfully inhibits this process, reducing the toxicity of irinotecan, but maintaining its antitumor activity against RTC cells. In addition, pectin itself reduced the viability of tumor cells, inducing their apoptosis and increasing the intracellular production of reactive oxygen species - the developing oxidative stress also reduced the viability of cancer cells. Thus, such pectin enhanced the cytotoxic and proapoptotic effects of irinotecan, exhibiting a synergistic effect with this drug. And finally, pectin exhibited a pronounced anti-inflammatory effect, preventing the adhesion (sticking together) of the invasive strain of Escherichia coli bacteria E. coli with colon cancer cells, which protected them from chemotherapy [13]. Oncologists from Wageningen University, the Netherlands, discovered the ability of pectins to increase the effectiveness of chemotherapy for colon cancer with the antitumor antibiotic doxorubicin. This effect is due to the fact that pectins bind and inhibit certain receptors (toll-like receptors2, TLR2), resulting in specifically inhibiting the pro-inflammatory pathway. This effect is most pronounced in pectins with a low degree of esterification. Such pectins interact with TLR2 due to electrostatic forces. Moreover, this effect does not depend on short-chain fatty acids produced by the intestinal microbiota [14-17]. Chemists from the National Institute of Natural Sciences, Indonesia, have developed a technology to increase the selectivity of the action of the cytostatic 5-fluorouracil (5-FU), which is widely used in the clinic for the treatment of colon cancer. Its disadvantage is its numerous side effects. The authors of the work created a transport form of 5-FU by combining it with pectin and chitosan (a polysaccharide of animal origin). This form has acquired the properties of more tightly adhering (contacting) with the mucous membrane of the large intestine at the site of its damage by tumor cells. The experiment showed that the resulting drug exhibited selective toxicity towards tumor cells [18]. Thus, the combination of modified pectin with various anticancer agents may represent an effective new strategy to overcome resistance in cancer patients [19,20]. The present studies were carried out to establish the effect of pectin modification on conventional chemotherapy.
Native pectin from sugar beet pulp was previously dispersed in a high-energy ball mill (SPEXSamplePrep 8000, 1425 rpm, the material of the balls and cells is tungsten carbide) for 10 min. The study of the antitumor effect of beet pectin on experimental animals was implemented according to the existing recommendations. An ethical review of the study materials was carried out by the Bioethics Committee of the Kyrgyz State Medical Academy (02/24/21). The maintenance of experimental animals was carried out in standard vivarium conditions. Experiments were conducted on 250 Wistar rats of both sexes weighing 160 g - 200 g, quarantined for 14 days. The rats were kept on a regular diet with free access to water and food, under normal temperature and light conditions. Walker’s transplanted tumor was transplanted under the skin of the thigh in a volume of 0.2 ml and a dilution of 1:1 area 199. Treatment was started 72 hours after the transplantation. Water-diluted pectin was injected with a per os probe once a day at a dose of 60 mg/kg for 10 days. On the 7th and 14th days from the start of treatment, the barbell was measured by three perpendicular to the size of the tumor. The result (volume) was expressed in the product of these quantities divided by two. The life expectancy of experimental animals was also taken into account. As criteria of morphological apoptosis of tumor cells and their drug pathomorphosis were used to assess the therapeutic effect [21]. Statistical processing of the results was carried out by nonparametric methods [22].
In the obtained pectin sample, the content of D-galacturonic acid was 83.0%, and the number of carboxyl and methoxyl groups was 14.8 and 4.6% respectively. The degree of esterification calculated from these data is 23.7. Determination of the molecular weight of pectin by a viscometric method using Ostwald capillary viscometer carried out according to the equation Kuhn−Mark−Howink.
[η] = kMa,
Where [η] is the intrinsic viscosity, M is the molecular weight, К = 1,1.10-5, and a = 1,22 – are constant coefficients for pectin.
The use of this equation makes it possible to determine a fixed value of the molecular weight of pectin. Mw = 15 kDa. In this article is the average molecular weight calculated based on the results of several measurements. Of pectin, particle size was 80 nm - 100 nm. pH of pectin suspension has been controlled as 5.6-6.0.
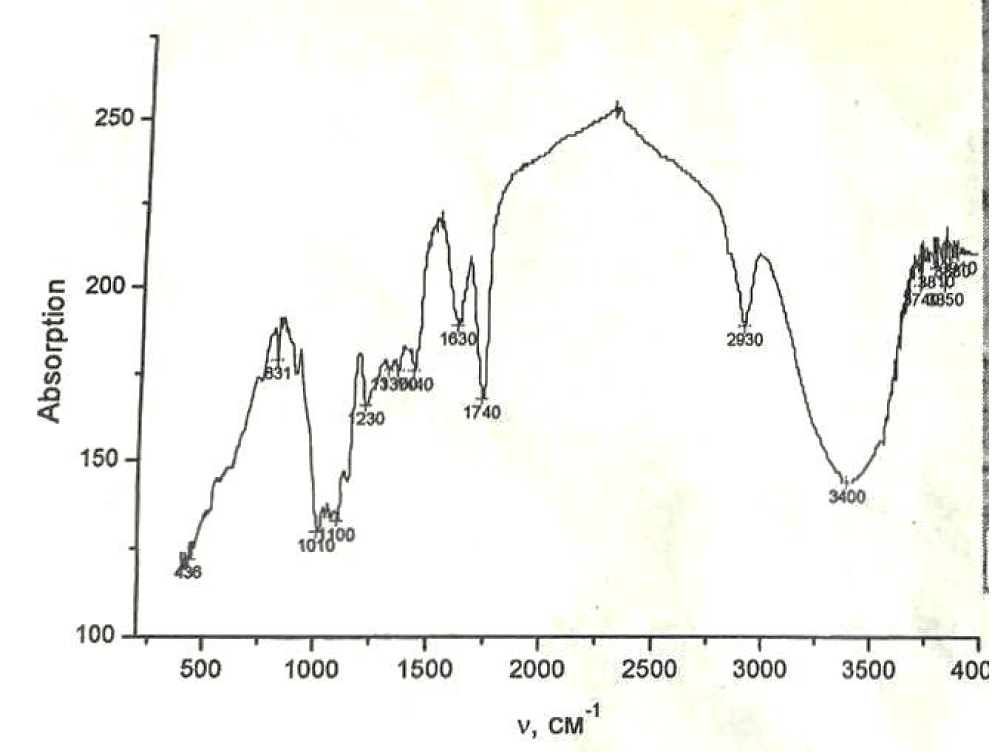
IR spectra of pectin show a wide absorption band at 3420 cm-1 − 3330 cm-1. These peaks correspond to the oscillation of hydroxyl groups involved in the formation of hydrogen bonds. In the low-frequency region, peaks are observed at 1750 cm-1 (v C=O) and asymmetric oscillations of ionized carboxyl groups vas (COO-) at 1630 cm-1. The maximum peak of 1420 cm-1 is related to the symmetrical oscillation of the group (COO). Weak absorption at 955 cm is caused by fluctuations in the OH group (Figure 1).
Figure 1: FTIR spectra of pectin (Specord 75 TIR spectrophotometer, 200 cm-1 - 4000 cm-1, KBr pellets).
Fifty male Wistar rats, weighing 120 g - 160 g, were randomly divided into the control group (n = 8), and experimental group (n = 6). Suspension of vegetative cells of the Walker carcinosarcoma 256 strain in exponential growth (1 x 106/mL) was injected subcutaneously into the inner femur surface of rats. The pectin in starch glue (Figure 2) was administered per os once per day in 400 mg/kg for 10 days from 72-h after transplantation to the necropsy day 21.
Figure 2: SEM images of the pectin suspension (CARL ZEISS MICROSCOPE EVO-40, Austria with Inca device).
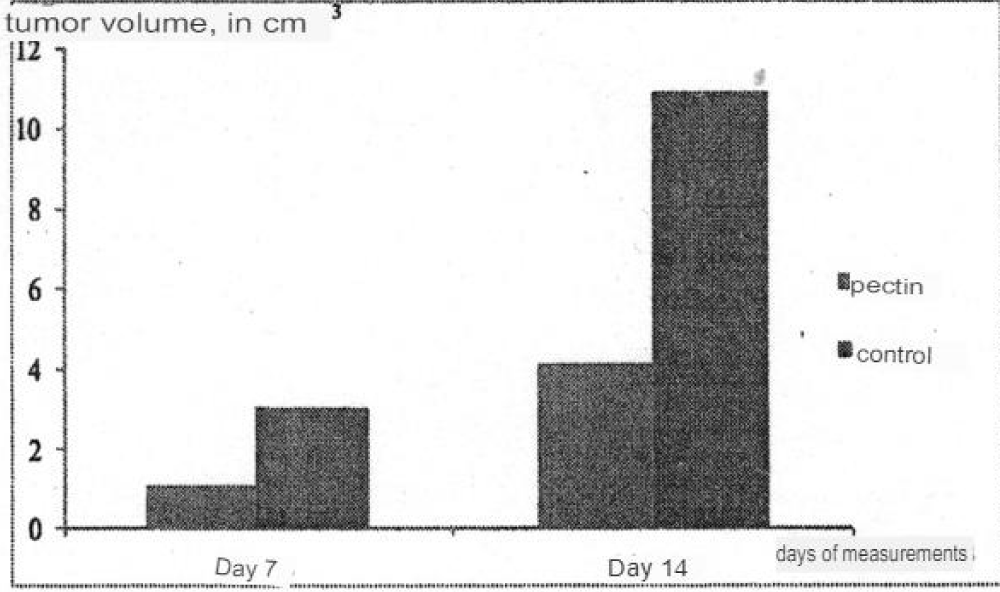
On the 7th day of the experiment, the tumor volume in the control was 3.89 ± 0.7 cm3, and in the experimental group - 1.02 ± 0.2 cm3. On the 14th day, this indicator in the control group was 10.93 ± 2.8 cm3, in the experimental group - 4.14 ± 2.0 cm3 (Figure 3). The difference in volumes reached a statistically significant level: on the 7th day p < 0.01, and on the 14th - p < 0.05 (the Wilkoxson-Mann-Whitney criterion). From these data, it follows that inhibition of tumor growth on the 7th day was 73.7% and on the 14th day it was 62.1%.
Figure 3: The influence of pectin on the Walker’s tumor growth.
Thus, pectin therapy of Walker’s tumor in several series of experiments consistently caused inhibition of its growth from 60 to 75%. As a rule, large values of this indicator were typical for the initial stage experiment. Then, as the deadlines increased, this indicator decreased. Although the majority of animals treated with pectin died from disease progression, the tumor regressed to 27.8% ± 8.9%. This affected the average life expectancy, which was 19.3 ± 1.9 days in the control group, and 38.56 ± 5.3 days in the experimental group. Accordingly, the increase in life expectancy was 99.8% (p < 0.01, the Wilcoxon-Mann-Whitney criterion).
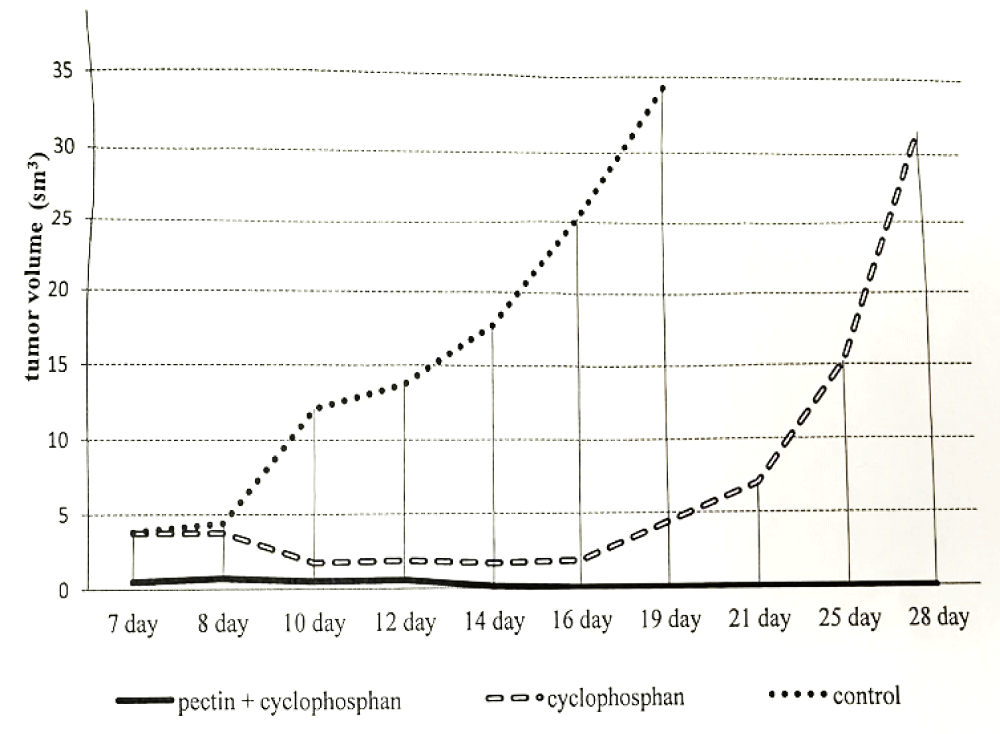
Simultaneously with the assessment of the effect of the study drug on the development of the primary tumor node, a study of its effect on the effectiveness of cytostatic chemotherapy was carried out. Cytostatics were administered in a regimen that caused moderate inhibition of the tumor process (30% - 60% inhibition of the growth of the primary tumor and metastases). A single intraperitoneal injection of cyclophosphamide at a dose of 25 mg/kg on the 5th day after transplantation was used as the main chemotherapy agent. Pectin was administered by a probe through the stomach, starting from the 2nd to the 13th day after tumor transplantation. In each series of experiments, the following groups were formed from the experimental animals: a control group, which received the appropriate solvent (water); a group that received only cytostatics and a group that received a cytostatic in combination with pectin. The experimental data are presented in Figure 4.
Figure 4: The kinetics of the Walker’s tumor growth.
In the period from the 10th to the 12th day, the difference between tumor sizes in the groups of animals receiving pectin with cyclophosphamide and cyclophosphamide was minimal. On the 10th day, in the pectin + cyclophosphamide group, the tumor size was 0.53 ± 0.19 cm3, and in the cyclophosphamide group - 1.75 ± 0.56 cm3. On the 14th day, 0.59 ± 0.27 cm3 and 1.88 ± 0.73 cm3, respectively. However, this difference reached a statistically significant value of p < 0.05 (Wilcoxon-Mann-Whitney test). The kinetic curves clearly show the effect of each drug and their combined use on tumor growth. The growth rate graph in the control group has a classic S-shaped curve with periods of accelerated growth in the initial and final periods of observation. The graph of tumor growth kinetics in the cyclophosphamide group also has a classic appearance. From the 7th to the 12th day, the growth rate decreases, then it is compared with the control until the 21st day. Later, the growth rate becomes higher than in the control.
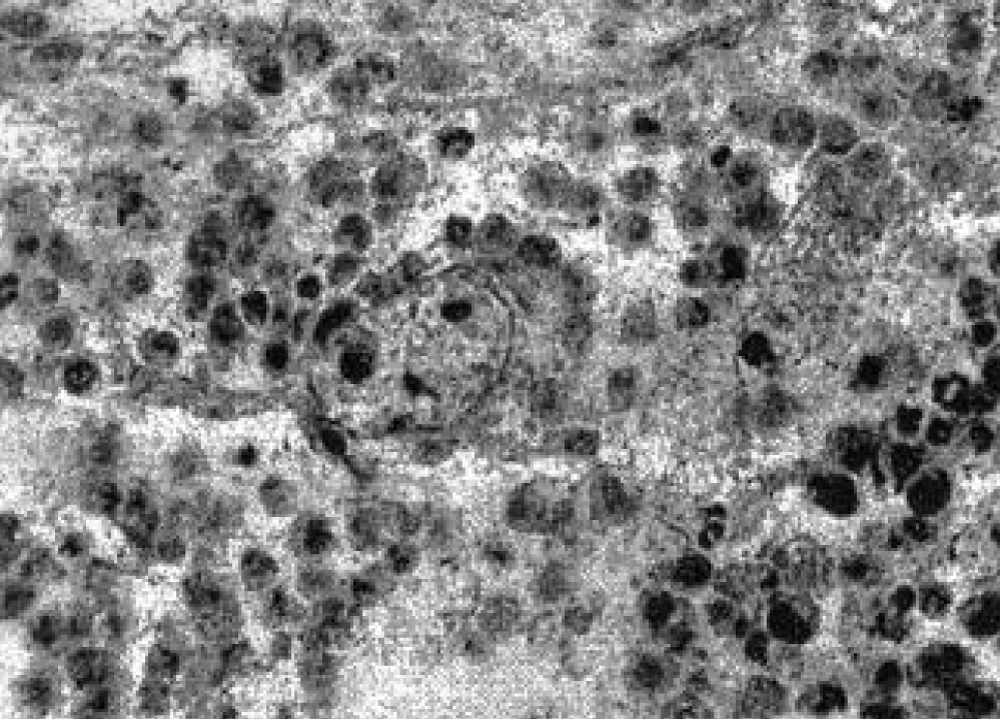
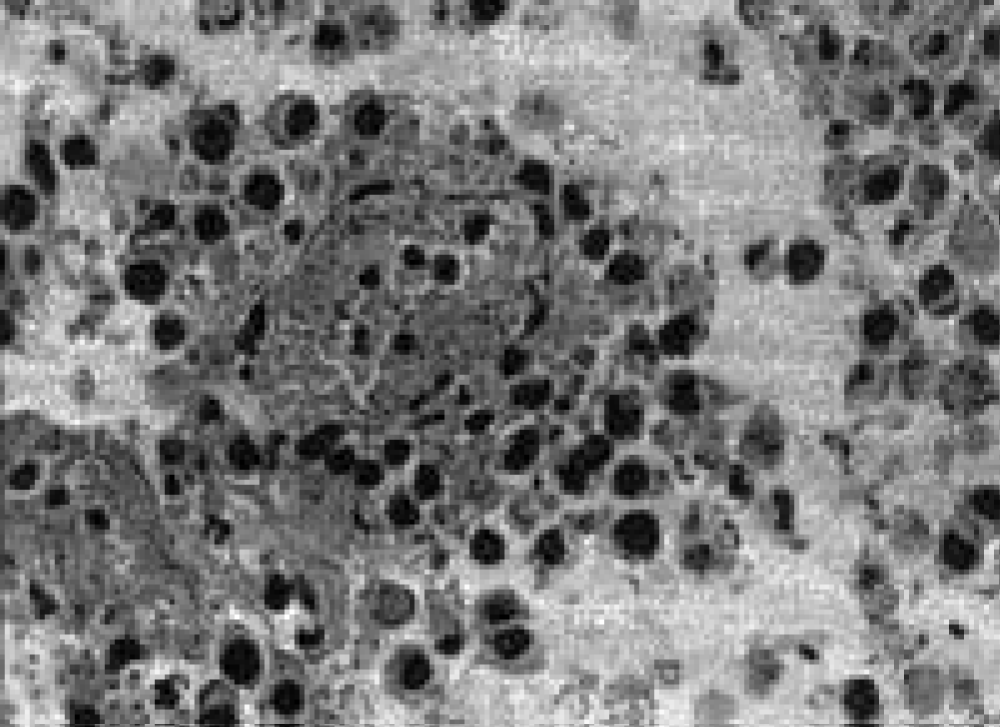
An assessment of drug pathomorphosis showed that its degree 2 was typical for the group of animals that received cyclophosphamide, and the 4th - for the group of animals that received pectin and cyclophosphamide. Detailed data and morphological studies carried out on the 20th day after transplantation revealed that the combination of cytostatic and pectin leads to pronounced apoptosis of tumor cells without the formation of foci of massive necrosis (Figure 5).
Figure 5: Apoptosis of tumor cells under the action of pectin and cyclophosphane.
In the period from the 10th to the 12th day, the difference between the size of the tumor in the groups of animals receiving pectin with cyclophosphane and cyclophosphane was minimal. On the 10th day in the “pectin + cyclophosphane” group, the tumor size was 0.53 ± 0.19 cm3, and in the cyclophosphane group - 1.75 ± 0.56 cm3. On the 14th day, respectively, 0.59 ± 0.27 cm3 and 1.88 ± 0.73 cm3. However, this difference reached a statistically significant value of p < 0.05 (Wilcoxon-Mann-Whitney criterion).
Life expectancy in the control group was 17.4 ± 1.99 days, and in the group “cyclophosphane” - 30 ± 0.97 days. Inhibition of tumor growth was 72.4% (p < 0.01). Since all the animals that received pectin in combination with cyclophosphane survived, and according to the screening rules, they were monitored for 90 days, this value was accepted as the average life expectancy of this group. Thus, the increase in life expectancy in the “pectin + cyclophosphane” group versus the “cyclophosphane” group was 200%.
The evaluation of drug pathomorphosis showed that its 2nd degree was characteristic of a group of animals treated with cyclophosphane, and the 4th – for a group of animals treated with pectin and cyclophosphane. Detailed data and morphological studies conducted on the 20th day after the transplant revealed that the combination of cytostatin and pectin leads to pronounced apoptosis of tumor cells without the formation of foci of massive necrosis (Figure 5).
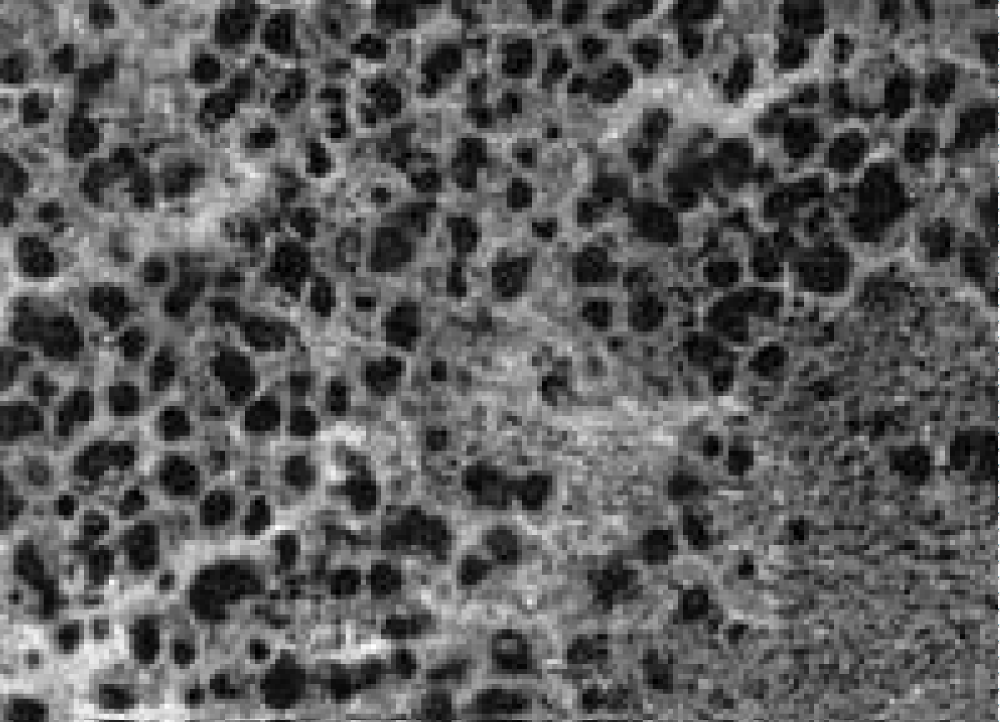
As can be seen in the photograph, almost all the nuclear material of cells in a state of apoptosis is highly fragmented (nuclear dust) and is preserved within the cytoplasm without disturbing the cytoplasmic membrane. Only a few tumor cells remained. In the entire mass of histological material examined, not a single mitosis could be detected. Subsequently, the tumor masses were replaced by surrounding tissues (connective, muscle, fat). During therapy with cyclophosphamide, on the 20th day after transplantation, viable tumor cells were found in large fields of necrotic masses, and the fields themselves were surrounded by a mass of intact tumor cells, some of which showed mitoses (Figure 6). Almost all visualized vessels contained complexes of tumor cells (Figure 7).
Figure 6: Tumor cells in the lumen of the vessel.
Figure 7: Necrosis fields and preserved tumor cells.
Significance
Monotherapy with pectin for Walker’s transplantable tumor in several series of experiments consistently inhibited its growth. Despite the fact that the life expectancy of animals in the experimental group was significantly higher than in the control group, the experimental picture, like a mirror, reflected the real state of affairs in practical oncology. In some patients, antitumor therapy has the desired effect in the form of stabilization of the process or regression of the tumor, but in a significant number of patients, it causes only a partial, temporary effect. The combined use of pectin and cyclophosphamide caused an enhancement effect at a dose of cyclophosphamide 4-5 times less than the therapeutic one. The tumor that appeared in the “pectin + cyclophosphamide” group eventually regressed, and all animals were completely cured. Since all animals that received pectin in combination with cyclophosphamide survived, and according to the screening rules, observation of them lasted 90 days, this value was accepted as the average life expectancy of this group. The increase in life expectancy in the pectin + cyclophosphamide group versus the cyclophosphamide group was 200%.
Thus, our data indicate that the combination of pectin and cyclophosphamide is synergistic because the amplification effect is obvious. To summarize, we can conclude that nanosized pectin is a promising drug for in-depth study since it satisfies one of the primary screening criteria. The increase in life expectancy of animals with Walker’s tumor that received it significantly exceeded the threshold level of 50%. The combined use of cyclophosphamide and pectin satisfies all three primary selection criteria:
The combined use of cyclophosphane and pectin satisfies all three criteria of primary selection:
1. 90% inhibition of tumor growth in the patient persisted for at least 7 days after the end of treatment.
2. The increase in the life expectancy of animals was significantly more than 50%.
3. More than 50% of the animals had no signs of a tumor process within 90 days after the end of treatment.
- Ovoldov Yu. Modern ideas about pectin substances. Bioorganic Chemistry. 2009; 35: 293-310.
- Zakaria NG, Rahman RA, Zaikal DNA, Yusoh M. Malaysian Journal of Fundamental and Applied Sciences. 2021; 17: 33-37.
- Azimova LB, Filatoov AV, Muchamedzhanova Myu, Turaev AC. Preparation and rheological properties of pectin, isolation using microwave radiation. Journal of Chemistry of Plant Raw Materials. 2023; 77-86.
- Pachmonova MO, Vasina SM, Naimova BK. Preparation and properties of pectins from food industry waste. Universum. 2023; 105: 14990. DOI10.32743/Unichem2023.
- Butova CN. Characteristics of pectins from non-traditional raw materials. Young Scientist. 2020; 22: C.424 - C. 426.
- Guryev AM. Chemical and pharmacological investigation of polysaccharides of higher plants and prospects of their use in the therapy of evil-qualitative neoplasms: dis ... Doctor of Pharmaceutical Sciences. Pyatigorsk. 2011; 297.
- Johnson KD, Glinskii OV, Mossine VV, Turk JR, Mawhinney TP, Anthony DC, Henry CJ, Huxley VH, Glinsky GV, Pienta KJ, Raz A, Glinsky VV. Galectin-3 as a potential therapeutic target in tumors arising from malignant endothelia. Neoplasia. 2007 Aug;9(8):662-70. doi: 10.1593/neo.07433. PMID: 17786185; PMCID: PMC1950436.
- Glinskii OV, Huxley VH, Glinsky GV, Pienta KJ, Raz A, Glinsky VV. Mechanical entrapment is insufficient and intercellular adhesion is essential for metastatic cell arrest in distant organs. Neoplasia. 2005 May;7(5):522-7. doi: 10.1593/neo.04646. PMID: 15967104; PMCID: PMC1501167.
- Nangia-Makker P, Hogan V, Honjo Y, Baccarini S, Tait L, Bresalier R, Raz A. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J Natl Cancer Inst. 2002 Dec 18;94(24):1854-62. doi: 10.1093/jnci/94.24.1854. PMID: 12488479.
- Sathisha UV, Jayaram S, Harish Nayaka MA, Dharmesh SM. Inhibition of galectin-3 mediated cellular interactions by pectic polysaccharides from dietary sources. Glycoconj J. 2007 Nov;24(8):497-507. doi: 10.1007/s10719-007-9042-3. Epub 2007 May 25. PMID: 17525829.
- Inohara H, Raz A. Effects of natural complex carbohydrate (citrus pectin) on murine melanoma cell properties related to galectin-3 functions. Glycoconj J. 1994 Dec;11(6):527-32. doi: 10.1007/BF00731303. PMID: 7696855.
- Dygai AM. Zueva EP, Razina TG. System of selection of natural compounds for use in oncological practice. Work experience of the Institute of Pharmacology with RAMS. Pacific Medical Journal. 2010; 10-15.
- Methodological recommendations for the preclinical study of drugs that have the ability to inhibit the process of metastasis and increase the effectiveness of cytostatic therapy of tumors. Guidelines for experimental (preclinical) study of new pharmacological substances. 2005; 674-682. (Russian)
- Sahasrabudhe NM, Beukema M, Tian L, Troost B, Scholte J, Bruininx E, Bruggeman G, van den Berg M, Scheurink A, Schols HA, Faas MM, de Vos P. Dietary Fiber Pectin Directly Blocks Toll-Like Receptor 2-1 and Prevents Doxorubicin-Induced Ileitis. Front Immunol. 2018 Mar 1;9:383. doi: 10.3389/fimmu.2018.00383. PMID: 29545800; PMCID: PMC5839092.
- Pérez-Loyola M. Modified pectins with activity against colon cancer. J Pharm Pharmacogn Res. 2022; 10: 616-651.
- Wong TW, Colombo G, Sonvico F. Pectin matrix as oral drug delivery vehicle for colon cancer treatment. AAPS PharmSciTech. 2011 Mar;12(1):201-14. doi: 10.1208/s12249-010-9564-z. Epub 2010 Dec 31. PMID: 21194013; PMCID: PMC3066368.
- Donadio JLS, Prado SBRD, Rogero MM, Fabi JP. Effects of pectins on colorectal cancer: targeting hallmarks as a support for future clinical trials. Food Funct. 2022 Nov 14;13(22):11438-11454. doi: 10.1039/d2fo01995g. PMID: 36314297.
- Liko A. In vitro evaluation of pectin-based drug delivery system for colorectal cancer-targeted drug delivery / Indonesia International Institute for Life Sciences. 2021; T202109065.
- Emran TB, Islam F, Mitra S, Paul S, Nath N, Khan Z, Das R, Chandran D, Sharma R, Lima CMG, Awadh AAA, Almazni IA, Alhasaniah AH, Guiné RPF. Pectin: A Bioactive Food Polysaccharide with Cancer Preventive Potential. Molecules. 2022 Oct 31;27(21):7405. doi: 10.3390/molecules27217405. PMID: 36364232; PMCID: PMC9657392.
- Hossein G, Halvaei S, Heidarian Y, Dehghani-Ghobadi Z, Hassani M, Hosseini H, Naderi N, Sheikh Hassani S. Pectasol-C Modified Citrus Pectin targets Galectin-3-induced STAT3 activation and synergize paclitaxel cytotoxic effect on ovarian cancer spheroids. Cancer Med. 2019 Aug;8(9):4315-4329. doi: 10.1002/cam4.2334. Epub 2019 Jun 13. PMID: 31197964; PMCID: PMC6675724.
- Manskikh VN, Manskikh BN. Morphological methods of verification and quantitative assessment of apoptosis. Bulletin of Siberian Medicine. 2004; 63-70.
- Gubler EV, Genkin AA. Application of nonparametric criteria of statistics in biomedical research. L. Medicine. 1973; 143.