More Information
Submitted: December 02, 2023 | Approved: December 09, 2023 | Published: December 11, 2023
How to cite this article: Letaief SB, Zemni I, Saadallah F, Ghalleb M, Sahraoui G, et al. A Rare Coexistence: Breast Cancer, Pheochromocytoma and Von Recklinghausen Disease. J Radiol Oncol. 2023; 7: 071-075.
DOI: 10.29328/journal.jro.1001057
Copyright License: © 2023 Letaief SB, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Von Recklinghausen’s disease; Breast cancer; Pheochromocytoma; Surgery
Abbreviated title: NF1: Type-1 Neurofibromatosis; ACR: American College of Radiology; BRCA: The Breast Cancer Gene Mutation; CT: Computed Tomography
A Rare Coexistence: Breast Cancer, Pheochromocytoma and Von Recklinghausen Disease
Sarra Ben Letaief1*, Ines Zemni1, Fatma Saadallah1, Montassar Ghalleb2, Ghada Sahraoui2, Mohamed Ali Ayadi1 and Tarek Dhieb1
1Department of Surgical Oncology, Salah Azaiez Institute, Faculty of Medicine of Tunis, University of Tunis El Manar, Tunis, Tunisia
2Department of Histology, Salah Azaiez Institute, Faculty of Medicine of Tunis, University of Tunis El Manar, Tunis, Tunisia
*Address for Correspondence: Sarra Ben Letaief, Department of Surgical Oncology, Salah Azaiez Institute, Faculty of Medicine of Tunis, University of Tunis El Manar, El Khadhra city, Tunis, 1003, Tunisia, Email: [email protected]
Breast cancer associated with type-1 neurofibromatosis is a rare clinical entity. These patients have a higher risk of developing various types of cancers, especially tumors derived from the embryogenic neural crest, such as pheochromocytoma. This publication aims to add to the literature a rare association between Type-1 Neurofibromatosis, breast cancer, and pheochromocytoma.
We present a rare case of a 51-year-old Tunisian woman with neurofibromatosis who was diagnosed with breast cancer and pheochromocytoma. The breast tumor was classified as T4b N1M0, and the discovery of the pheochromocytoma was incidental to thoracic-abdominal-pelvic CT. She underwent surgery to remove the adrenal gland and was referred to medical oncologists to receive chemotherapy for her breast cancer. Type-1 Neurofibromatosis disorder is a benign disease but can expose patients to numerous neoplasms. The challenging diagnosis at an early stage can worsen the prognosis and make medical care more difficult.
Neurofibromatosis type 1 (NF1), also known as Von Recklinghausen’s disease, is a rare neuroectodermal autosomal dominant disorder that touches 1 in 3000-4000 individuals, mainly affects the skin and the nervous system, is characterized by cafe-au-lait spots and multiple neurofibromas [1].
This disease exposes a high risk of developing tumors derived from the neural crest, such as pheochromocytoma.
The association of breast cancer and NF1 has been rarely reported in the literature; we report one case of a Tunisian woman diagnosed with NF1, breast cancer, and pheochromocytoma incidentally.
The aim of this case report is to point out the possibility of the adrenal mass revealed during the staging of breast cancer and to highlight the importance of correct metastatic status, which has a significant influence on the treatment.
This case was treated at the Salah Azaiez Institute in 2023.
For reference data, we used PubMed using the keywords mentioned.
We report a case of a 51-year-old woman from the Sub-Saharan region with no personal or family medical history who consulted a local doctor for a left breast lump. The general examination showed an average blood pressure profile, multiple tan spots in different sizes and shapes associated with numerous lentiginous lesions in the trunk, face, and four limbs (Figure 1).
Figure 1: Picture of the trunk showing the multiple lentiginous lesions, café-au-lait spots, and the disparity of breast volume.
The breast exam showed a maligned 5cm mass with inflammatory manifestations of the left breast and axillary lymphadenopathy.
The breast imaging showed a 5 cm malignant mass located in the upper and external quadrant classified ACR 5; the biopsy and the immunohistopathology confirmed the diagnosis of luminal B, grade 2, invasive ductal carcinoma; Estrogen and progesterone receptors were positive, HER (human epidermal growth factor receptor) was negative and Ki67 (marker of tumor proliferation) was 30%.
CT scan done to rule out a metastasis revealed a heterogenous mass measuring 30*27 mm, suspicious of malignancy located in the left adrenal gland; further investigations showed that the Levels of metanephrine and normetanephrine in blood and urine were high, presented in table number 1 (Table 1), confirming the presence of a pheochromocytoma.
| Table 1: Metanephrine and normetanephrine levels in the bloodstream and urine. | ||
| Biochemical indicator | Level measured (nmol/L) | Reference level (nmol/L) |
| Metanephrine (blood) | 3.85 | < 1.07 |
| Normetanephrine (blood) | 0.79 | < 0.33 |
| Metanephrine (urine) | 678 | < 120 |
| Normetanephrine (urine) | 1739 | < 280 |
The multidisciplinary meeting decided to perform a left adrenalectomy first and then start neoadjuvant chemotherapy for her locally advanced breast cancer.
An alpha-blocker was administered for two weeks before the pheochromocytoma surgery; during the intervention, the heart rate and blood pressure were high, reaching 150 contractions per minute and 240 mmHg through the dissection of the adrenal gland. However, as soon as the blood support of the gland was interrupted through a ligature, all the hemodynamic parameters were normalized.
The postoperative course was simple, and the patient was discharged in three days.
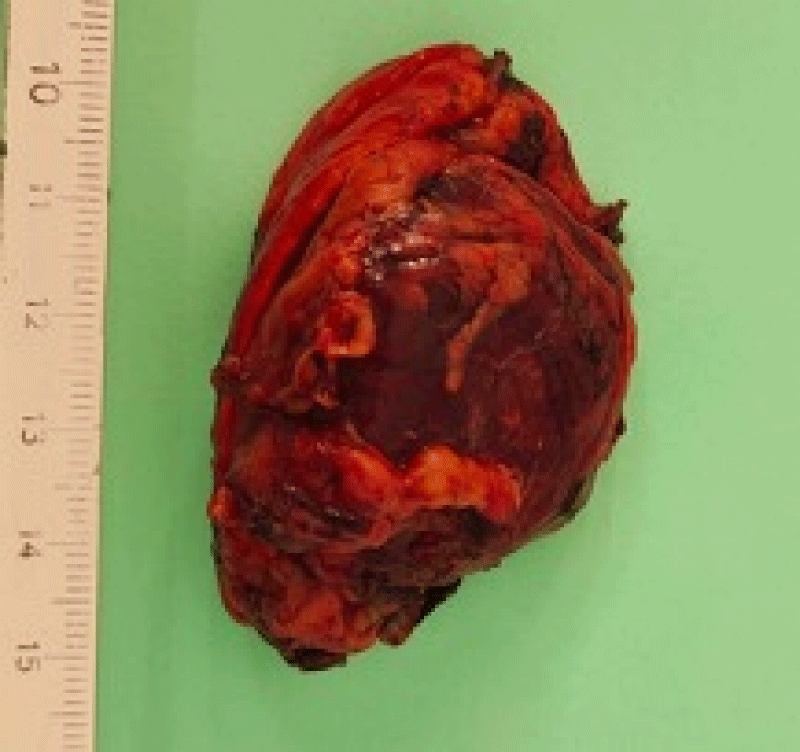
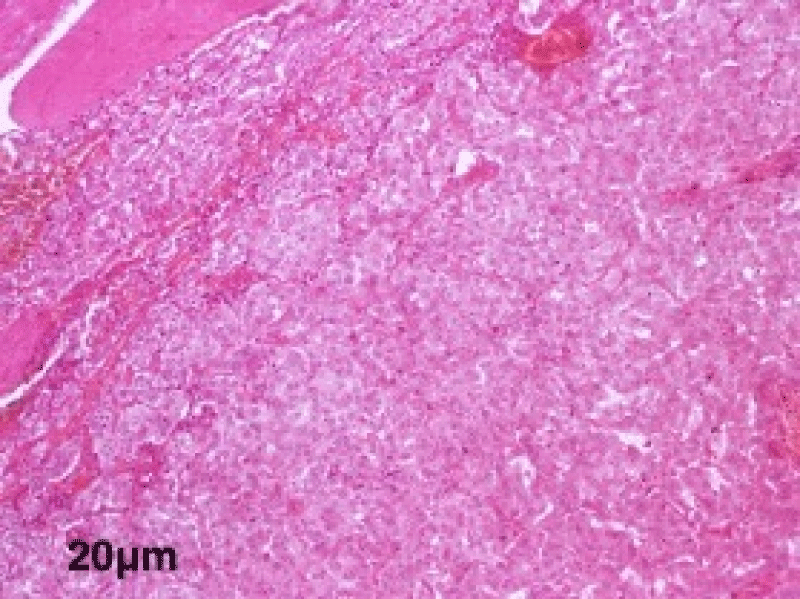
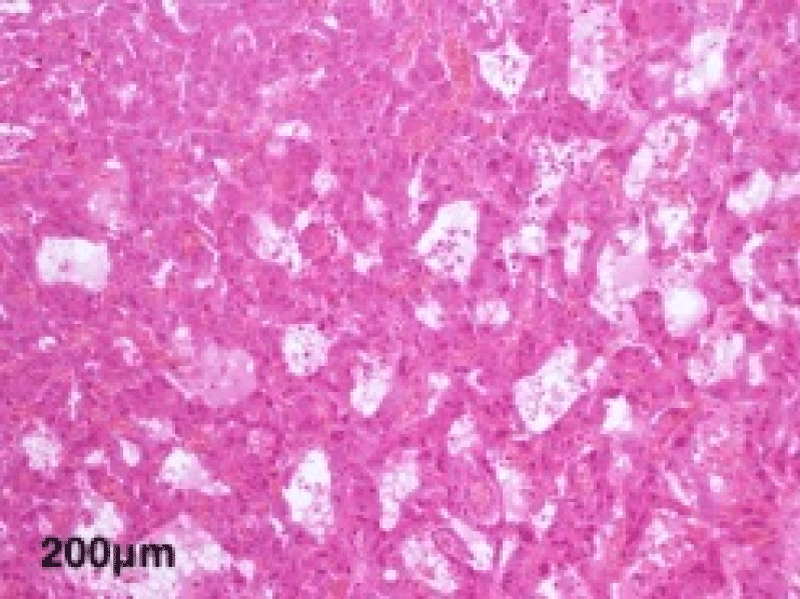
The histological study of the adrenalectomy specimen (Figures 2,3), confirmed the diagnosis of pheochromocytoma (Figures 4,5).
Figure 2: The macroscopic examination; sagittal section of the adrenal gland.
Figure 3: The macroscopic view of the adrenal gland before taking the samples.
Figure 4: A low magnification view showing the encapsulated tumor proliferation of diffuse architecture of giant cells at the microscopic examination.
Figure 5: A high magnification view showing the tubular architecture of large cells with abundant eosinophilic cytoplasm and monomorphic nuclei.
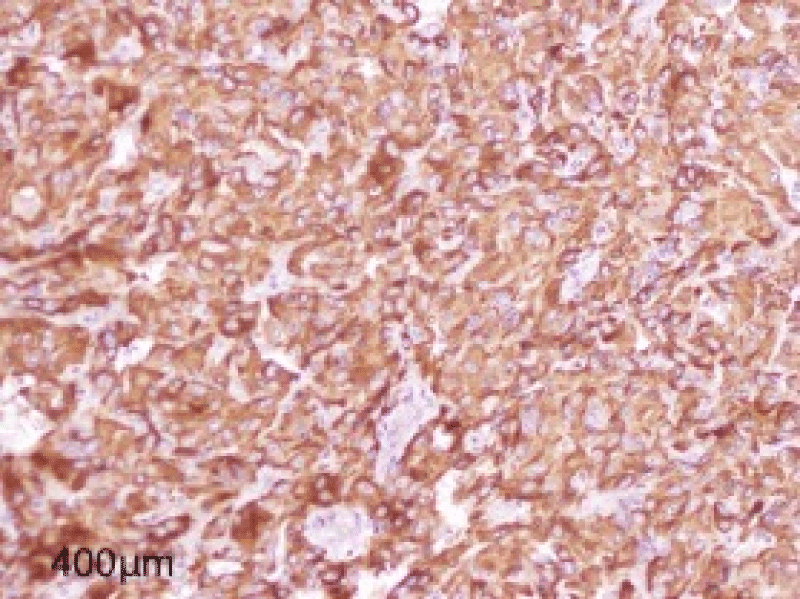
The immunohistochemistry study showed Chromogranin synaptophysin was positive and cytokeratin Melan A was negative (Figure 6).
Figure 6: Figure showing Intense and diffuse cytoplasmic immunostaining by Chromogranin.
After her recovery from the surgery, she was referred to the clinical oncology department to start chemotherapy; due to the inevitable use of anthracycline drug, a cardiovascular check-up was indicated and revealed to be normal. After finishing the neoadjuvant treatment, the patient went back to the surgery department and underwent a mastectomy.
The histological study of the mastectomy showed a partial response; a tumor remnant of 43 mm and four lymph nodes were positive out of 16 nodes. Afterward, she received radiotherapy and adjuvant endocrine therapy.
NF1 is a complex neuroectodermal disorder, described for the first time in 1849 by Von Recklinghausen; it is characterized by the presence of numerous soft tissue neurofibromas, cafe-au-lait macules, a high rate of tumors in the nervous system such as Malignant peripheral nerve sheath tumors (MPNST), optic pathway glioma, and less frequent neoplasms of neuroectodermal or mesenchymal origin, such as pheochromocytoma [1].
Pheochromocytoma occurs in 0.1% - 5.7% of patients with NF1; it produces high amounts of catecholamine, resulting in tachycardia and hypertension [2].
It is found in 4% of patients with adrenal incidentaloma, despite no symptoms or signs suggesting pheochromocytoma, similar to our case report.
The NF1 gene is mapped to chromosome 17q11.2; it acts as a tumor suppressor.
The gene responsible for the specific association between NF1 and pheochromocytoma remains unknown [3].
Association with breast cancer
Since 1970, few case reports have been published. Brasfield and Das Gupta published the first 5 cases [4], and Murayama et al. reported 37 cases of breast cancer associated with NF1 [5].
In 2019, Suarez-Kelly et al. published a Meta-analysis along with a systematic review of literature, a total of 4178 women diagnosed with BC and NF1. They found that this category of patients under 50 years old has five times more chances of developing breast cancer when compared to the general population, with more advanced disease, and may have an increased BC-related mortality [7].
An observation of a familial form of breast cancer and NF1 was reported by Salemis NS et al. in 2010 [8].
Since the location of the two genes BRCA1 and NF1 are in the same chromosome 17 [9], a concomitant mutation of the two genes is very likely. It could confer a hereditary character to this association [10].
According to a recent study conducted by Dischinger, Patrick S et al. in 2018, NF1 gene deletion is associated with NF1-ER-FOXA1-AR networks and causes endocrine-resistant breast cancer in both NF1-related breast cancer and sporadic breast cancer, highlighting a functional link between neurofibromin and the estrogen receptor [11].
These genetic alterations can alter their Product, specifically neurofibromin, a protein that controls cell differentiation and proliferation, inhibiting the p21ras activation pathway. As a result, it puts it on permanent activity and chaotic cell proliferation [9,12].
Most of the cases were diagnosed at an advanced stage, the same scenario for our case; it is probably due to the presence of cutaneous fibromatosis in the trunk in most of the patients. Therefore, it makes it difficult to palpate the breast lumps in a self-examen, which could lead to confusion and delayed diagnosis, which lead to a poorer prognosis and higher mortality rate.
Yap, Yoon-Sim, et al. compared 18 women with NF1 and breast cancer and with 7132 women diagnosed with breast cancers without NF1 and concluded that NF1 patients have more aggressive breast cancer with an inferior overall survival and that is related to germline NF1 mutations in cooperation with somatic mutations in TP53, KMT2C, and other genes [13].
The importance of correct staging in breast cancer
Breast cancer often spreads to bones, the liver, and lungs. Metastatic spread of breast carcinoma to adrenal glands is very rare.
Benign adenomas constitute the most common group of adrenal tumors, and pheochromocytoma can be easily interpreted as adrenal metastases, as they are relatively large, heterogeneous with central necrosis, and display significant contrast enhancement on CT scans. Unlike metastases, however, most pheochromocytomas are well-shaped and have smooth margins [14].
The dosage of methoxylated derivatives (used in this case) can be beneficial to making the proper diagnosis.
Pheochromocytoma may be fatal if not diagnosed. It requires surgery as a priority, even if there are no symptoms or signs of pheochromocytoma.
To the best of our review of the literature, only a few papers reported the rare combination with NF1, pheochromocytoma, and breast cancer (Turkey, Japan, and Korea) [15-17]. These patients should be classified as a high-risk group and encouraged to undergo breast ultrasonography or breast MRI early in life.
According to Evans DGR et al. study, 26.5% of NF1 patients who survived for 20 years from the first breast cancer diagnosis developed cancer in the contralateral side [18]. Such risk justifies considering prophylactic mastectomy of the unaffected breast in these patients.
The authors have completed the CARE Checklist for this case report, attached as supplementary material.
NF1 is commonly a benign disease, but the reported cases of cancer-related to this disorder can worsen the prognosis due to the advanced stage of diagnosis and the conflicted clinical presentation. It is mandatory to differentiate benign adrenal lesions from metastasis in order to make the specific management prior to adrenalectomy.
Statement of ethics
Ethics approval and consent to participate.
The approval to publish this case report is not required from the medical and ethics committee of the Salah Azaiez Institute, however; this work is done with all due respect to the code of ethics under the supervision of the same committee.
Written informed consent for publication
Written informed consent was obtained from the patient to publish this case report and any accompanying images.
Authors’ contribution
BS, FS: Data collection, drafted the manuscript; SS, RD: Data collection review of the literature; IZ, MA: A review of the literature and draft of the manuscript; TD: Drafted the manuscript.
Data availability statement: The data supporting our findings were taken from the patient’s folder, further enquiries can be directed to the corresponding author.
Acknowledgment
The paramedical team of the surgery department of the institute Salah Azaiez for their continuous efforts.
- Dannenberg H, van Nederveen FH, Abbou M, Verhofstad AA, Komminoth P, de Krijger RR, Dinjens WN. Clinical characteristics of pheochromocytoma patients with germline mutations in SDHD. J Clin Oncol. 2005 Mar 20;23(9):1894-901. doi: 10.1200/JCO.2005.07.198. PMID: 15774781.
- Neumann HP, Bausch B, McWhinney SR, Bender BU, Gimm O, Franke G, Schipper J, Klisch J, Altehoefer C, Zerres K, Januszewicz A, Eng C, Smith WM, Munk R, Manz T, Glaesker S, Apel TW, Treier M, Reineke M, Walz MK, Hoang-Vu C, Brauckhoff M, Klein-Franke A, Klose P, Schmidt H, Maier-Woelfle M, Peçzkowska M, Szmigielski C, Eng C; Freiburg-Warsaw-Columbus Pheochromocytoma Study Group. Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med. 2002 May 9;346(19):1459-66. doi: 10.1056/NEJMoa020152. PMID: 12000816.
- Pacak K, Linehan WM, Eisenhofer G, Walther MM, Goldstein DS. Recent advances in genetics, diagnosis, localization, and treatment of pheochromocytoma. Ann Intern Med. 2001 Feb 20;134(4):315-29. doi: 10.7326/0003-4819-134-4-200102200-00016. PMID: 11182843.
- Brasfield RD, Das Gupta TK. Von Recklinghausen's disease: a clinicopathological study. Ann Surg. 1972 Jan;175(1):86-104. doi: 10.1097/00000658-197201000-00015. PMID: 4621893; PMCID: PMC1355163.
- Murayama Y, Yamamoto Y, Shimojima N, Takahara T, Kikuchi K, Iida S, Kondo Y. T1 Breast Cancer Associated with Von Recklinghausen's Neurofibromatosis. Breast Cancer. 1999 Jul 25;6(3):227-230. PMID: 11091721.
- Sharif S, Moran A, Huson SM, Iddenden R, Shenton A, Howard E, Evans DG. Women with neurofibromatosis 1 are at a moderately increased risk of developing breast cancer and should be considered for early screening. J Med Genet. 2007 Aug;44(8):481-4. doi: 10.1136/jmg.2007.049346. Epub 2007 Mar 16. PMID: 17369502; PMCID: PMC2597938.
- Suarez-Kelly LP, Yu L, Kline D, Schneider EB, Agnese DM, Carson WE. Increased breast cancer risk in women with neurofibromatosis type 1: a meta-analysis and systematic review of the literature. Hered Cancer Clin Pract. 2019 Mar 25;17:12. doi: 10.1186/s13053-019-0110-z. PMID: 30962859; PMCID: PMC6434896.
- Salemis NS, Nakos G, Sambaziotis D, Gourgiotis S. Breast cancer associated with type 1 neurofibromatosis. Breast Cancer. 2010 Oct;17(4):306-9. doi: 10.1007/s12282-009-0119-7. Epub 2009 May 23. PMID: 19466510.
- Riccardi V.M. Genetic alterations and growth factors in the pathogenesis of Von Recklinghausen neurofibromatosis. Neurofibromatosis. 1989; 2: 266-73.
- Ceccaroni M, Genuardi M, Legge F, Lucci-Cordisco E, Carrara S, D'Amico F, Greggi S, Scambia G. BRCA1-related malignancies in a family presenting with von Recklinghausen's disease. Gynecol Oncol. 2002 Sep;86(3):375-8. doi: 10.1006/gyno.2002.6757. PMID: 12217765.
- Dischinger, Patrick S et al. “NF1 deficiency correlates with estrogen receptor signaling and diminished survival in breast cancer.” NPJ breast cancer vol. 4 29. 30 Aug. 2018, doi:10.1038/s41523-018-0080.
- Pinson S, Créange A, Barbarot S, Stalder JF, Chaix Y, Rodriguez D, Sanson M, Bernheim A, D'incan M, Doz F, Stoll C, Combemale P, Kalifa C, Zeller J, Teillac-Hamel D, Lyonnet S, Zerah M, Lacour JP, Guillot B, Wolkenstein P. Neurofibromatose 1: recommandations pour la prise en charge [Neurofibromatosis 1: recommendations for management]. Ann Dermatol Venereol. 2001 Apr;128(4):567-75. French. PMID: 11395662.
- Yap YS, Munusamy P, Lim C, Chan CHT, Prawira A, Loke SY, Lim SH, Ong KW, Yong WS, Ng SBH, Tan IBH, Callen DF, Lim JCT, Thike AA, Tan PH, Lee ASG. Breast cancer in women with neurofibromatosis type 1 (NF1): a comprehensive case series with molecular insights into its aggressive phenotype. Breast Cancer Res Treat. 2018 Oct;171(3):719-735. doi: 10.1007/s10549-018-4851-6. Epub 2018 Jun 21. PMID: 29926297.
- Ctvrtlik F, Koranda P, Tichy T. Adrenal disease: a clinical update and overview of imaging. A review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158(1):23-34. doi: 10.5507/bp.2014.010. Epub 2014 Feb 20. PMID: 24621966.
- Demirpence MM, Bahceci M, Dolek D, Salgur F, Gorgel A, Tutuncuoglu P. A Very Rare Assocıatıon; Coexıstence of Breast Cancer, Pheochromocytoma and Neurofıbromatosıs Type 1 in a Female Patıent. International Journal of Case Reports in Medicine. 2013 (2013); Article ID 511990, DOI: 10.5171/2013.511990.
- Lee YH, Kwon MJ, Park JH, Jeong SJ, Kim TH, Jeong HW, Lee SH. Neurofibromatosis Type 1 with the Development of Pheochromocytoma and Breast Cancer. Intern Med. 2020 Jul 1;59(13):1665-1669. doi: 10.2169/internalmedicine.4148-19. Epub 2020 Apr 9. PMID: 32269189; PMCID: PMC7402965.
- Jeong JW, Jeon S, Yu TY, Kim HS, Cho CG. An Extraordinary Case of Pheochromocytoma with Breast Cancer in a Patient with Neurofibromatosis Type 1. Chonnam Med J. 2020 May;56(2):154-155. doi: 10.4068/cmj.2020.56.2.154. Epub 2020 May 25. PMID: 32509566; PMCID: PMC7250663.
- Evans DGR, Kallionpää RA, Clementi M, Trevisson E, Mautner VF, Howell SJ, Lewis L, Zehou O, Peltonen S, Brunello A, Harkness EF, Wolkenstein P, Peltonen J. Breast cancer in neurofibromatosis 1: survival and risk of contralateral breast cancer in a five country cohort study. Genet Med. 2020 Feb;22(2):398-406. doi: 10.1038/s41436-019-0651-6. Epub 2019 Sep 9. Erratum in: Genet Med. 2019 Oct 8;: PMID: 31495828; PMCID: PMC7000349.