More Information
Submitted: February 20, 2024 | Approved: March 12, 2024 | Published: March 13, 2024
How to cite this article: Deng R, Duan M, Ma X, Chen J, Zhang H, et al. Effect of TAK242 on MCP-1 and TGF-β in COPD Rats. J Radiol Oncol. 2024; 8: 014-021.
DOI: 10.29328/journal.jro.1001060
Copyright License: © 2024 Deng R, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Chronic obstructive pulmonary disease; Pulmonary fibrosis; TAK242; Animal model
Abbreviated title: COPD: Chronic Obstructive Pulmonary Disease; SD rats: Sprague-Dawley rats; MCP-1: Monocyte Chemotactic Protein-1; TGF-β: Transforming Growth Factor-β; ECM: Extracellular Matrix; EMT: Epithelial-tumour-mesenchymal Transition; TAK242: Resatorvid; NF-kB: Nuclear Transcription Factor-κB; IL-1: Interleukin-1
Effect of TAK242 on MCP-1 and TGF-β in COPD Rats
Ruicheng Deng1#, Mingyu Duan2#, Xiaoyong Ma3, Juanxia Chen4, Huifang Zhang4, Meifang Liu4, Jian Chen2 and Lijun Chen4* 
1Second Clinical Medical College of Ningxia Medical University, Yinchuan 750004, China
2Clinical Medical College of Ningxia Medical University, Yinchuan 750001, China
3Department of Traditional Chinese Medicine, General Hospital of Ningxia Medical University 750001, China
4Department of Respiratory and Critical Care Medicine, the Second Affiliated Hospital of Ningxia Medical University, Yinchuan 750001, China
#Co-first authors
*Address for Correspondence: Lijun Chen, Department of Respiratory and Critical Care Medicine, The Second Affiliated Hospital of Ningxia Medical University (The First People’s Hospital of Yinchuan), No.2 Liqun West Street, Xingqing District, Yinchuan, Ningxia 750001, China, Email: [email protected]
Objective: To investigate the mechanism of MCP-1 and TGF-β regulation by TAK242 in COPD rats.
Methods: Thirty-six SD rats were randomly divided into normal, COPD control, and TAK242 groups. The normal group was freely fed, and the other groups used the method of fumigation plus lipopolysaccharide tracheal drip to establish an experimental animal model of COPD. After successful modeling, each experimental group received 0.9% NaCl solution and corresponding drugs by intraperitoneal injection for 7 d. After drug administration, lung function was examined; pathological changes in lung tissue were observed by light microscopy with hematoxylin-eosin staining; mRNA expression of MCP-1 and TGF-β was detected by q-PCR; and protein expression of MCP-1 and TGF-β in lung tissue was detected by Western blot and IHC, TGF-β protein expression in rat lung tissue.
Results: Compared with the normal group, rats in the COPD control group showed signs and symptoms of COPD, decreased lung function, and increased expression of MCP-1 and TGF-β. The TAK242 group showed decreased expression of MCP-1 and TGF-β compared to the COPD control group.
Conclusion: MCP-1, and TGF-β played a crucial role in the early stage of COPD fibrosis. TAK242 could ameliorate airway inflammation and inhibit the progression of COPD lung fibrosis in pre-existing rats in COPD model rats.
COPD due to long-term inhalation of smoke and other pollutants, caused by inflammatory reactions, lung infections, or deposition of interstitial immune complexes in the lung and other factors, inducing the production of fibrotic cytokines, which is the main cause of the formation of chronic pulmonary fibrosis [1-3]. Pulmonary fibrosis is also a major challenge in the diagnosis and treatment of lung disease. It is therefore important to delay the progression of pulmonary fibrosis and to find effective treatments. As the pathogenesis of pulmonary fibrosis is better understood, there is increasing evidence that inflammation is also a recognized factor in the pathogenesis of pulmonary fibrosis [4]. Inflammatory responses such as immune inflammatory cells and cytokines play an important role in the development of pulmonary fibrosis. The inflammatory response in pulmonary fibrosis is caused by the increased expression of monocytes and macrophages in lung tissue; MCP-1 and TGF-β are two important causes leading to the infiltration of monocytes and macrophages into lung tissue [5,6]. MCP-1 is one of the major inflammatory factors involved in lung injury and plays a key role in exacerbating the progression of COPD pulmonary fibrosis. As a member of the inflammatory chemokine family, MCP-1 mediates the infiltration of a wide range of inflammatory cells in COPD lung fibrosis, with the number of monocytes and macrophages being directly proportional to the degree of interstitial fibrosis [7]. TGF-β is the major cytokine involved in the pathogenesis of pulmonary fibrosis in COPD. The inflammatory environment enhances oxidative stress-induced TGF-β expression [8], while TGF-β promotes cellular hypertrophy, ECM accumulation, and EMT. Among the many regulators of EMT, TGF-β is considered an important factor in pulmonary fibrosis [9]. Although many studies have elucidated the formation mechanism of pulmonary fibrosis, the intrinsic influence of inflammatory mediators in the disease remains to be further investigated and confirmed. Inflammation inhibition has the characteristics of multi-pathway and multi-target, which has certain advantages in the prevention and treatment of pulmonary fibrosis, and plays an important role in the treatment of pulmonary fibrosis, with comprehensive therapeutic advantages in anti-inflammatory, antioxidant, and delaying pulmonary fibrosis [10-12]. Among them, TAK242 belongs to the toll-like receptor inhibitors, which affect the NF-kB signaling pathway, which belongs to the typical inflammatory pathway, and inhibition of which has the functions of reducing inflammation, improving the degree of fibrosis to regulate immunity and antioxidant [13,14], etc. In this study, based on previous studies and the efficacy of known drugs, we established a COPD model and observed the expression of pulmonary fibrosis factors MCP-1 and TGF-β in lung tissues to explore the therapeutic role and mechanism of TAK242 in the early stage of pulmonary fibrosis in COPD.
Experimental animals
Basic information: Thirty-six healthy SPF-grade male SD rats with an average body mass of (180±10) g were provided by the Experimental Animal Centre of Ningxia Medical University (SYXK Ning 2020-0001), randomly divided into the COPD group and the normal group, with 13 rats in the normal group and 23 rats in the COPD group, all housed in a clean SPF grade environment. At the time of modeling, 3 rats each from the normal and COPD groups were randomly selected to verify the success of the model, and then the COPD group was randomly divided into a COPD control group (10 rats) and a TAK242 group (10 rats).
Reagents and instruments: Lipopolysaccharide (Sigma: L2880), TAK242(Solarbio), Taishan brand cigarettes (Shandong China Tobacco Company), TIANGEN RNA extraction kit (DP431), reverse transcription kit (Takara: RR036A), Q-PCR kit (Takara: RR820A), primers (Shanghai Sangong Co., Ltd.); homemade staining boxes (70 cm×55 cm×35 cm) and a homemade staining box (70 cm×55 cm×35 cm). (70 cm×55 cm×35 cm), smoke generator (Buxco, USA), small animal lung function meter (Buxco, USA), UV-visible spectrophotometer (Thermo, USA), fluorescence quantitative PCR (Bio-Rad, USA).
Methods
COPD rat model preparation: Lipopolysaccharide (200 μg/time) was injected into the trachea of rats in the COPD group on days 1, 14, 28 and 42; the rats were placed in a home-made poisoned box for passive smoking on days 2-13, 15-27 and 29-41 (2 times a day, each time for 60 min, with an interval of > 8 h between the two times, and each time for 10 cigarettes); the modeling was performed for a total of 42 d [15-17]. The rats in the normal group received no special treatments. At the end of the modeling, 3 rats from each of the COPD and normal groups were randomly selected and the airflow limitation of the COPD group rats was assessed using a small animal spirometer; The rats were anesthetized with sodium pentobarbital, and then the chest was opened after execution under anesthesia, and the lung tissues were collected for HE staining, which verified that the lung function and histopathological changes of the COPD group rats were consistent with the characteristics of COPD, and confirmed that the COPD rat model was successfully established. The rat model of COPD was successfully established.
Intervention program: 1mg/ml Lipopolysaccharide solution: Prepare according to the following recipe, dispense, and place in a 4 °C refrigerator.
Reagent Dose
Lipopolysaccharide 10 mg
0.9% saline 10 ml
1 mL TAK242 working solution
Reagent Dose
TAK242 5mg
DMSO 50 μL
PEG300 400 μL
Tween80 50 μL
ddH2O 500 μL
Marginal notes: Take 50 μL of 102 mg/ml of clarified DMSO stock solution and add it to 400 μL of PEG300, mix well to make it clarified; add 50 μL of Tween80 to the above system, mix well to make it clarified; and then continue to add 500 μL of ddH2O to fix the volume to 1 mL.
Normal group: free feeding, no intervention.
COPD control group: COPD rats were injected intraperitoneally with the same volume of saline once a day for 7 consecutive days.
TAK242 group: TAK242 was administered to COPD rats by intraperitoneal injection at 6 mg/kg once a day for 7 consecutive days.
Pulmonary function measurements in rats: Rats were anesthetized with 2% pentobarbital (50 mg/kg) by intraperitoneal injection, the trachea was incised in the middle of the neck, and the tracheal tube was inserted and placed in the body box. Total lung capacity (TLC), 200 ms rate (FEV200/FVC), and 100 ms rate (FEV100/FVC) were measured using a small animal spirometer.
Rat lung tissue sampling and pathological observation: Rats were killed by air embolization, and the left lung was removed from the open chest and immersed in 14% paraformaldehyde fixative for 24 h. The lung was rinsed with phosphate buffer solution and then serially sectioned by conventional paraffin embedding and stained with HE. The upper lobe of the right lung of rats was placed in a freezing tube, frozen in liquid nitrogen and then stored in a refrigerator at -80 ℃.
Determination of MCP-1 and TGF-β mRNA in rat lung tissues: Total RNA was extracted from frozen right lung tissues according to the instructions of the TIANGEN RNA Kit, and the OD260/280 was measured by UV spectrophotometer at 1.8-2.0. cDNA was synthesized according to the instructions of the Reverse Transcription Kit, and the mRNA concentration was determined using a qPCR kit. The reaction system consisted of 20 μL, 10 μL TB Green Premix, 0.8 μL forward primer, 0.8 μL reverse primer, 2 μL DNA template, and 6.4 μL of sterilized water, and the reaction conditions were as follows: pre-denaturation at 95 ℃ for 30 s, and cycling for 40 times according to the following procedure: denaturation at 95 ℃ for 5 s, annealing at 57 ℃ for 30 s, and extension at 72 ℃ for 1 min. The relative expression of MCP-1 and TGF-β was determined by the 2-ΔΔCt method using the release curve of the instrument and ACTB as an internal reference.
Western blot detection of MCP-1, TGF-β protein expression: The rats in normal, COPD, and TAK242 groups were killed under anesthesia, then the chest was opened, the right lung tissues were quickly taken out and put into liquid nitrogen, the rat lung tissues were taken out and ground, and the protein immunoblotting method was applied for protein quantification of rat lung tissues for MCP-1 and TGF-β. Total tissue protein was extracted according to the instructions of the kit, and protein quantification was performed by the BCA method. Take 40μg of protein and add the sample buffer to denature for use. Preparation of SDS-PAGE gel, concentrated gel 100V voltage electrophoresis 40min, separated gel 120V voltage electrophoresis 1h, 200mA constant current wet rotation 80min, the protein will be transferred to the PVDF membrane, Li Chun red staining to observe the bands, TBST wash the membrane, 5% skimmed milk TBST closure of 1. 5h, add a primary antibody incubated at room temperature for 2h, TBST wash the membrane, add a secondary antibody reaction of 1h, TBST wash the membrane, ECL luminous reagent luminescence, film exposure development and fixation. TBST washed membrane, ECL luminous reagent luminescence, film exposure, development, and fixation. The images were captured and the results were scanned for absorbance using gel image analysis software, and the ratio of absorbance of MCP-1 and TGF-β protein bands to GADPH was used as the amount of MCP-1 and TGF-β protein expression. The differences in MCP-1 and TGF-β protein expression in rat lung tissues of the two groups were compared.
Immunohistochemical staining: After paraffin embedding and sectioning, tissue sections were routinely deparaffinized and dehydrated, 3% hydrogen peroxide (H202) deionized water was closed to peroxidase, the reaction was carried out for 10 minutes, and rinsed in distilled water for 5 minutes × 3 times. In citrate buffer, microwave heat repair, boiling twice, PBS rinse, 5 min × 3 times. Reagent A (blocking solution, blue liquid) was added dropwise, incubated for 20 min at room temperature, decanted, do not wash. Add the corresponding primary antibody dropwise, overnight at 4 °C, 30 min at room temperature, and rinse in PBS, 5 min × 3 times. Drops of reagent B (the corresponding secondary antibody working solution, yellow liquid), room temperature incubation for 15 minutes, PBS rinse, 5 minutes × 3 times, drops of reagent C (horseradish enzyme-labeled streptavidin working solution, orange liquid), room temperature incubation for 20 minutes, PBS rinse, 5 minutes × 3 times. DAB color development (microscopic observation of the color of the color to terminate the color development), the tap water sufficiently washed, and hematoxylin restaining 30 seconds. Dewatering, transparency, sealing. The positive cells were determined by the presence of brownish-yellow granular deposits in the cytoplasm or nucleus. Comparison of the semiquantitative and localized expression of MCP-1 and TGF-β proteins in the lung tissues of four groups of rats.
Statistical methods
The experimental data were expressed by the mean standard deviation (x̄ ± S), they were processed by SPSS statistical software. One-way ANOVA was used to compare multiple samples. With a difference of p < 0.05 being statistically significant.
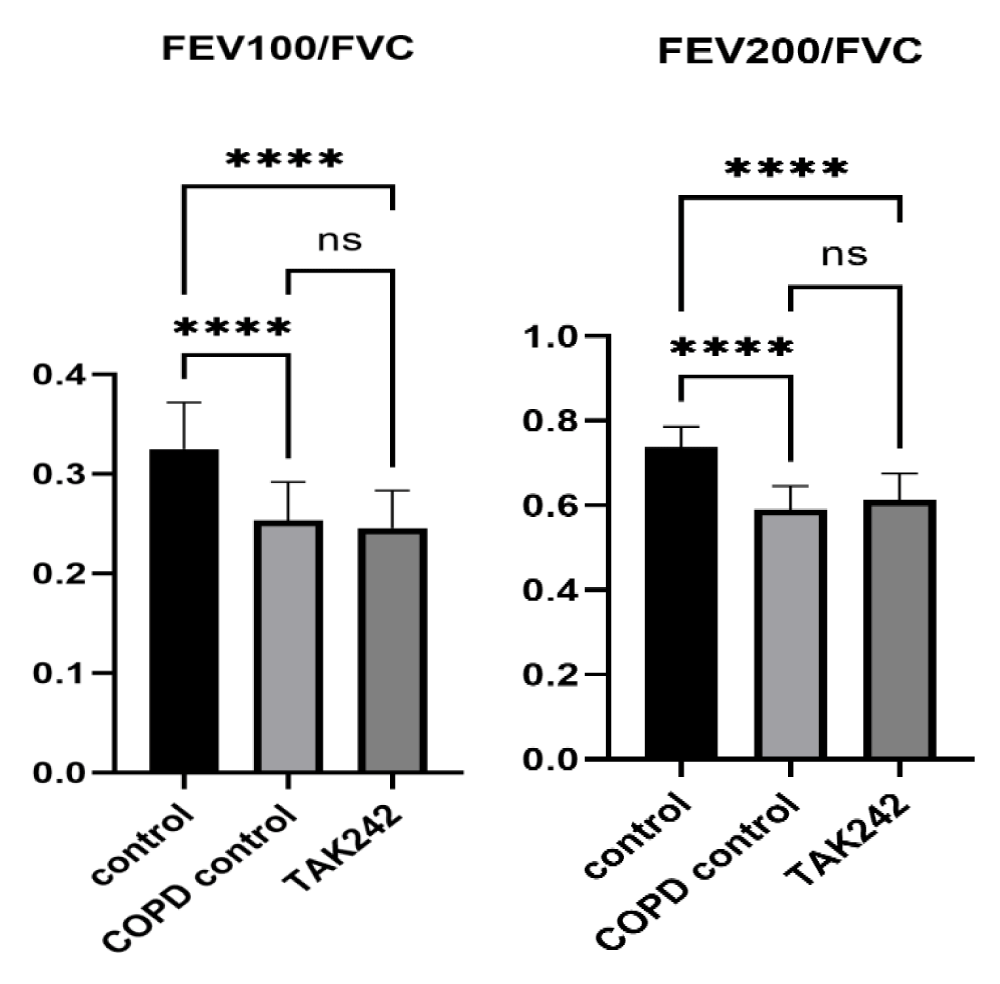
Significant effect on lung function in COPD rats
In order to verify the effect of COPD on the lung function of rats, we tested FEV100/FVC and FEV200/FVC parameters, and the results showed that there was a significant difference in FEV100/FVC and FEV200/FVC between rats in the control group and rats in the COPD group (p < 0.05), but there was no difference in the improvement of lung function of rats in the TAK242 group (p > 0.05) (Figure 1).
Figure 1: Comparison of lung function parameters between groups of rats, FEV100/FVC of control group vs. COPD group, ****:p < 0.05,ns: p > 0.05
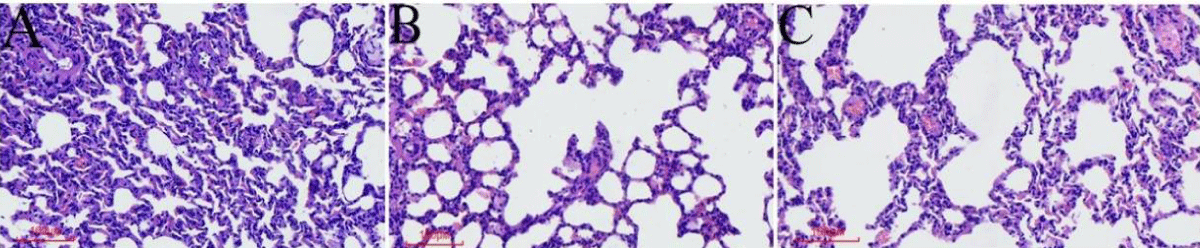
Figure 2: The general condition of the rats and histopathological changes in the lungs (A). HE staining of lung tissue in the control group. (B) HE staining of lung tissue of the COPD group. (C) HE staining of lung tissue in the TAK242 group.
Different interventions have significant effects on the general condition and histopathological changes in the lungs of COPD rats
The rats in the normal group had normal activity and diet throughout the modeling period, shiny hair, good mood, and normal respiration; HE of lung tissue showed that the bronchial walls were regular without inflammatory cell infiltration and the alveolar structure was normal without pathological expansion (Figure 2A). Compared with the normal group, rats in the COPD group gradually reduced their activities and food consumption with the prolongation of the exposure time to LPS+ smoke, their fur became dull and pale yellow, and their mood was depressed, accompanied by congestion of the eyeballs and the appearance of nasal secretions; HE of lung tissues showed mucus secretion and inflammatory cell infiltration of bronchial tubular lumen, submucosal glandular hyperplasia, disorganization of alveolar structure, and enlargement of alveolar lumen coalescing into pulmonary pustules (Figure 2B). Compared with the COPD control group, the TAK242 group showed a significant reduction in bronchiolar luminal mucus and inflammatory cell infiltration, and the rats’ activity and diet improved significantly, with bright fur color and good mood. The HE of the lung tissue showed significantly less mucus secretion and inflammatory cell infiltration in the bronchial lumen, and the changes in the alveoli and glands were not obvious (Figure 2C).
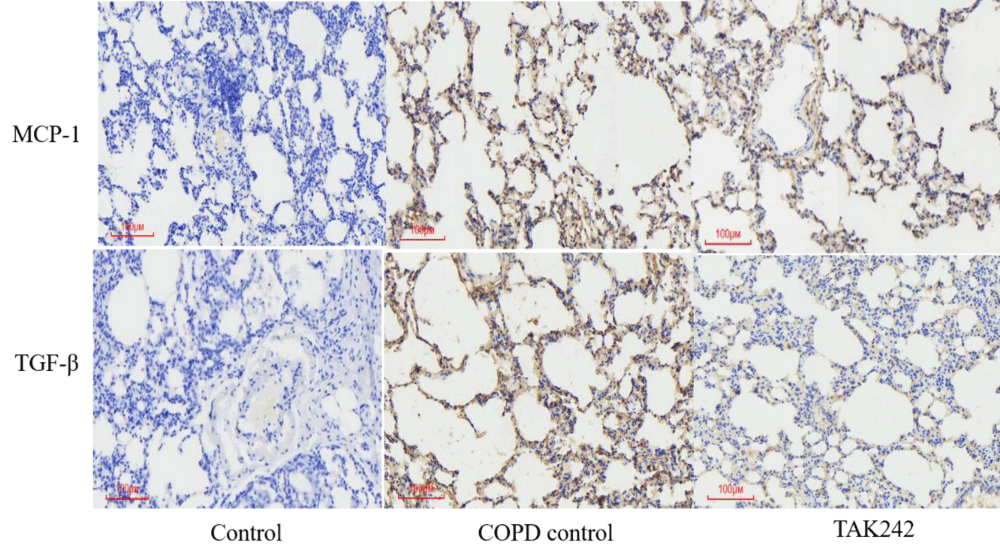
Different interventions regulate the expression of MCP-1 and TGF-β in lung tissue
We used IHC to detect MCP-1 and TGF-β protein expression in rat lung tissues, and the results showed that the expression of each protein index was high in the COPD group and lowest in the normal group. Among them, the expression of MCP-1 protein in the lung tissue of rats in the COPD control group was significantly higher than that in the normal group; the expression of TGF-β protein in the lung tissue of rats in the COPD group was significantly higher than that in the control group; (p < 0.05, Figure 3).
Figure 3: Expression of MCP-1 and TGF-β in lung tissue from different groups.
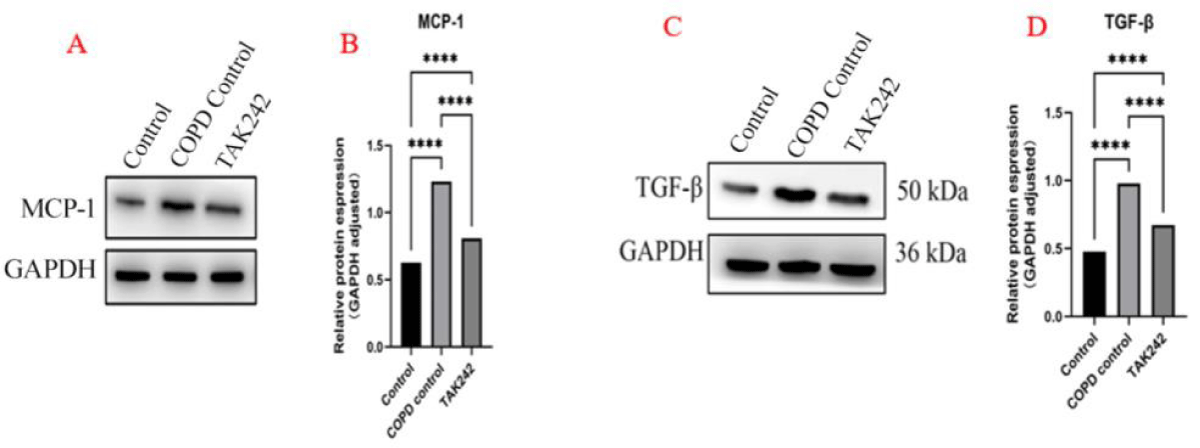
Changes in MCP-1 and TGF-β protein levels in lung tissues of different intervention groups
We used WB to semiquantitatively detect the results of the above IHC experiments, and the results showed that the expression trend of MCP-1 and TGF-β was consistent with the results of the IHC experiments, and the statistical analysis of the grey value suggested that the expression of MCP-1 and TGF-β was high in the COPD group, and the expression level of the normal group was low (p < 0.05, Figure 4).
Figure 4: Intervention regulates the expression of MCP-1, and TGF-β in lung tissue. (A). MCP-1 expression levels in each group. (B) MCP-1 WB statistics, ****p < 0.05. (C). TGF-β expression levels in each group. (D) TGF-β WB statistics,****p < 0.05.
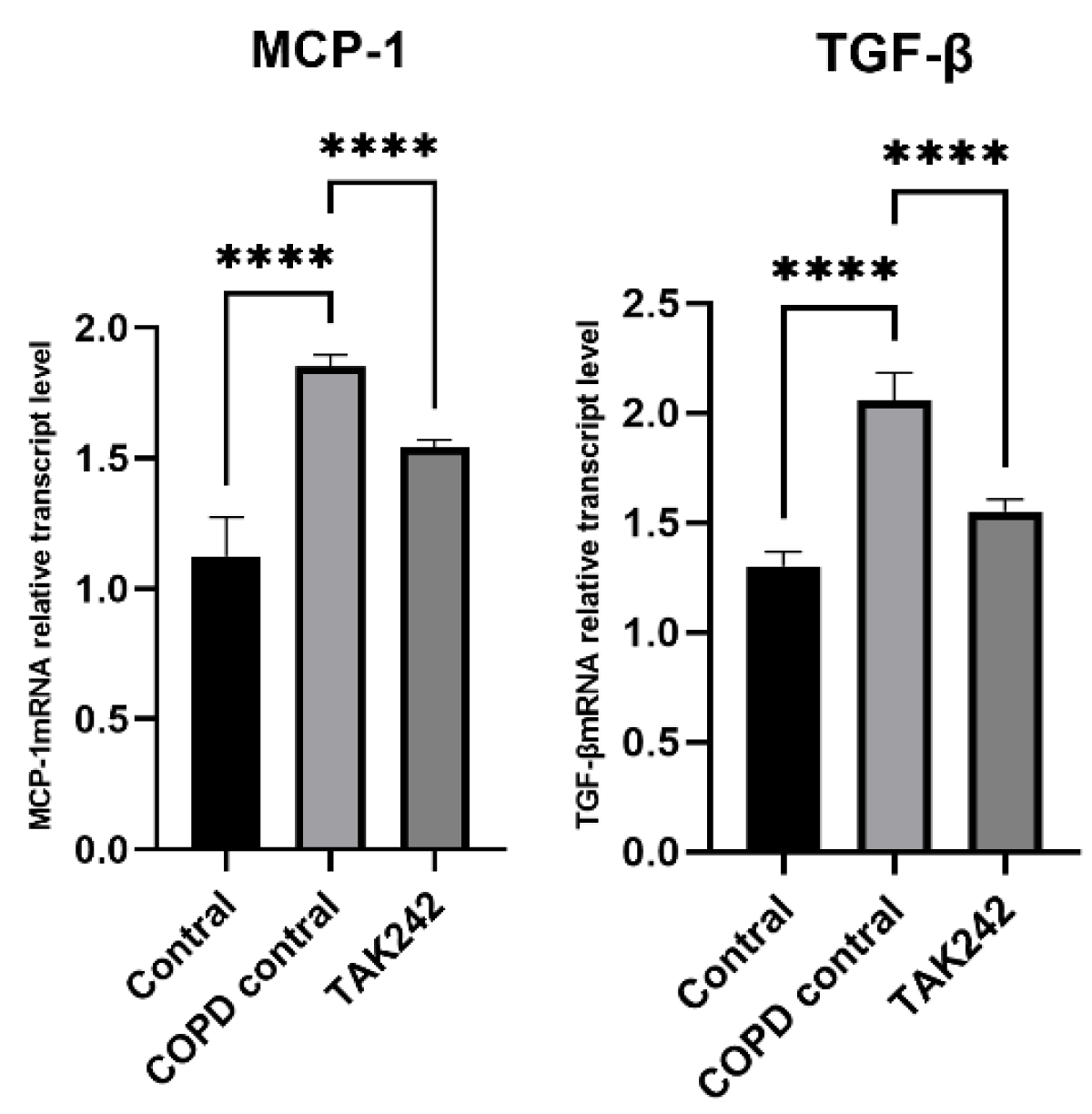
Different interventions regulate the relative expression of MCP-1 and TGF-β mRNA in rat lung tissue
There were differences in the levels of MCP-1 and TGF-β in the lung tissues of rats in each group. The levels of MCP-1 and TGF-β in the lung tissues of rats in the COPD control group were significantly higher than those in the normal group (p < 0.05). (Figure 5).
Figure 5: Relative expression of MCP-1 and TGF-β mRNA in plasma of rats regulated by different interventions. (A) MCP-1 mRNA expression level in lung tissue of each group. (B) TGF-β mRNA expression levels in lung tissues of each group.
Pulmonary fibrosis is one of the complications of COPD [1,18], with mild clinical manifestations in the early stage and the following pathological features: early lesions are mainly confined to the alveolar wall, the alveolar lumen is filled with fluid and cellular exudates, and the structure of the alveoli is blurred and disappears; then there is the destruction of fibrous tissue, disorganization of the lung structure, and the gradual development of interstitial fibrosis. Numerous studies have shown the presence of inflammatory cell infiltration, elevated cytokines, and increased levels of inflammatory mediators in pulmonary fibrosis [19] and in the early stage of pulmonary fibrosis, monocyte/macrophage infiltration is present in all lung tissues [20]. Infiltrating monocytes/macrophages destroy lung structures by releasing cytokines, inflammatory mediators, and oxygen free radicals, accelerating the process of lung tissue sclerosis. In this study, after the success of the COPD model, MCP-1 and TGF-β were found to be elevated in rat lung tissue, but the HE of lung tissue did not show signs of fibrosis, but the elevation of MCP-1 and TGF-β was positively proportional to chronic lung fibrosis, confirming that there is an obvious causal relationship between COPD and lung fibrosis. Our use of TAK242 to inhibit toll-like receptors resulted in a significant decrease in MCP-1 and TGF-β and had the function of delaying lung fibrosis. In our study, we found that TAK242 did not significantly improve lung function in rats, suggesting that inhibition of this inflammatory pathway was not able to reverse alveolar damage. Other studies have shown that inflammation is a key factor in the pathogenesis of lung fibrosis [21]. Although COPD is a recurrent inflammatory process, many cytokines are involved in the inflammatory response, such as interleukin-6 (IL-6), TGF-β, MCP-1, etc [7,22,23]. They play different roles and form a complex cytokine network. Among them, MCP-1 and TGF-β are closely associated with the progression of fibrosis.MCP-1, as a member of the inflammatory chemokine superfamily, can be released by a wide range of cells and is low expressed under physiological conditions. In contrast, under pathological conditions such as hyperglycemia and oxidized lipoproteins, thylakoid and endothelial cells can stimulate MCP-1 gene expression. On the one hand, high levels of MCP-1 an autocrine or paracrine stimulate the expression of IL-1 and TGF-β produced by alveolar cells and exacerbate endothelial cell injury. On the other hand, alveolar cells can be directly activated to produce myofibrillar proteins, leading to interstitial fibrosis. Activated macrophages can release reactive oxygen radicals, inflammatory mediators, and growth factors that promote the increase and deposition of ECM (extracellular matrix) [24], ultimately leading to the development of pulmonary arterial sclerosis. Evidence suggests that MCP-1 is a key factor in the early inflammatory process of atherosclerosis and also plays a central role in renal pathology, promoting the development of renal fibrosis and the progression of diabetic nephropathy [25]. Transforming growth factor-β, which is predominantly distributed in the lung, is a central factor in the complex cytokine network associated with the pathogenesis of pulmonary fibrosis and regulates tissue damage and repair under normal conditions. Overproduction of TGF-β in patients with pulmonary fibrosis is associated with chronic fibrosis. In addition, TGF-β expression is associated with oxidative stress, which is increased by prolonged hyperglycemia [26]. TGF-β mediates the TGF-β/Smad signaling pathway, which promotes ECM aggregation and decreases the activity of enzymes that degrade ECM, ultimately leading to interstitial lung fibrosis. In addition, TGF-β induces autophagy of renal tubular epithelial cells and promotes apoptosis of renal tubular epithelial cells, leading to tubular injury. Meanwhile, TGF-β is associated with the formation of autophagic proteins, leading to pulmonary capillary fibrosis [27]. High expression of TGF-β upregulates and enhances the transcription of MCP-1 through the nuclear transcription factor-κB (NF-κB) signaling pathway. Meanwhile, high expression of MCP-1 can activate the p38-MAPK signaling pathway via inflammatory response factors released by activated monocytes/macrophages to produce TGF-β, which promotes fibrosis and leads to lung lesions [28]. Studies have shown that when recombinant TGF-β is present in organisms, MCP-1 levels increase, while macrophages synergizing with MCP-1 can in turn stimulate the production of TGF-β and promote the deposition of ECM, ultimately leading to interstitial lung fibrosis [29]. Thus, TGF-β and MCP-1 directly or indirectly interact to form a vicious circle, exacerbating the inflammatory response and promoting the progression of pulmonary fibrosis. Therefore, MCP-1 and TGF-β play a crucial role in the development of fibrosis, suggesting that MCP-1 and TGF-β can be used as biomarkers to assess the development of pulmonary fibrosis. TAK242 can effectively inhibit the production of TGF-β and MCP-1, after which it can effectively reduce the local aggregation of monocytes/macrophages, reduce the deposition of ECM, and delay the progression of pulmonary fibrosis. TAK242 and its activated components have important biological effects such as anti-inflammatory, immunomodulatory, anti-tumor, and antioxidant effects [30]. This experiment showed that the use of TAK242 over a period of time could alleviate some pathological changes in the early stage of pulmonary fibrosis, such as basement membrane thickening and ECM accumulation [13]. Lung fibrosis can occur in the rat COPD model, and EMT is an important mechanism for the development and progression of interstitial lung fibrosis [31]. TAK242 was found to be effective in reducing the inflammatory response in the lung and ameliorating the inflammatory situation in rats in vivo. In terms of mechanism study, the expression of MCP-1 and TGF-β was significantly upregulated in the COPD control group, and TAK242 could inhibit the expression of MCP-1 and TGF-β in lung tissues. Our results suggest that TAK242 plays a role in the prevention of COPD lung fibrosis by inhibiting, attenuating, and treating MCP-1 and TGF-β, key factors in inflammation and fibrosis. In addition, we found that TAK242 reduced inflammatory cell infiltration in HE-stained sections. In recent years, a growing body of new evidence has demonstrated that TAK242 has broad therapeutic efficacy and comprehensive therapeutic value with fewer side effects. Given these advantages, TAK242 is a promising chemical agent. There are some possible limitations to this study. First, this experiment was limited to animals only, which may lead to bias in clinical trials. TAK242 has a variety of effects such as antioxidative stress, anti-inflammation, and immunomodulation, and only a small part of anti-inflammatory cytokines and inflammatory effects were shown in this study. Therefore, the mechanism of the protective effect of TAK242 on COPD lung fibrosis needs further investigation.
The present study demonstrated that TAK242 has a promising protective effect against COPD-induced lung fibrosis. These beneficial effects were closely associated with the downregulation of MCP-1 and TGF-β expression, which is important for the development of new strategies for the prevention and treatment of pulmonary fibrosis in COPD.
Compliance statement
This research has been conducted in accordance with ethical standards and regulatory guidelines, and has received full approval from the Laboratory Animal Ethical and Welfare Committee of the Laboratory Animal Center at Ningxia Medical University. The Laboratory Animal Ethical and Welfare Committee of Laboratory Animal Center has reviewed and approved the research protocols, ensuring the protection and welfare of any human participants, animals, or data involved. The study adheres to the principles outlined in relevant regulations. Approval for the study was granted under the protocol or project license/approval number IACUC-NYLAC-2022-067, with the approval dated March 2022.
Project funding (project number)
1. Yinchuan science and technology plan project(2021-SF-001);
2. Ningxia Autonomous region scientific and technological innovation leader(2021GKLRLX03);
3. Ningxia Autonomous Region Science and Technology Benefit People project(2022CMG03033,2021CMG03020);
4. Autonomous region health appropriate technology promotion project(2022-NWSY-020).
Author contributions
All authors contributed to researching the data, discussing the content, writing the text, and reviewing or editing the manuscript before submission.
- Barnes PJ. Small airway fibrosis in COPD. Int J Biochem Cell Biol. 2019 Nov;116:105598. doi: 10.1016/j.biocel.2019.105598. Epub 2019 Sep 6. PMID: 31499176.
- Rao W, Wang S, Duleba M, Niroula S, Goller K, Xie J, Mahalingam R, Neupane R, Liew AA, Vincent M, Okuda K, O'Neal WK, Boucher RC, Dickey BF, Wechsler ME, Ibrahim O, Engelhardt JF, Mertens TCJ, Wang W, Jyothula SSK, Crum CP, Karmouty-Quintana H, Parekh KR, Metersky ML, McKeon FD, Xian W. Regenerative Metaplastic Clones in COPD Lung Drive Inflammation and Fibrosis. Cell. 2020 May 14;181(4):848-864.e18. doi: 10.1016/j.cell.2020.03.047. Epub 2020 Apr 15. PMID: 32298651; PMCID: PMC7294989.
- Beghé B, Cerri S, Fabbri LM, Marchioni A. COPD, Pulmonary Fibrosis and ILAs in Aging Smokers: The Paradox of Striking Different Responses to the Major Risk Factors. Int J Mol Sci. 2021 Aug 27;22(17):9292. doi: 10.3390/ijms22179292. PMID: 34502194; PMCID: PMC8430914.
- Savin IA, Zenkova MA, Sen'kova AV. Pulmonary Fibrosis as a Result of Acute Lung Inflammation: Molecular Mechanisms, Relevant In Vivo Models, Prognostic and Therapeutic Approaches. Int J Mol Sci. 2022 Nov 29;23(23):14959. doi: 10.3390/ijms232314959. PMID: 36499287; PMCID: PMC9735580.
- Puukila S, Lawrence MD, De Pasquale CG, Bersten AD, Bihari S, McEvoy-May J, Nemec-Bakk A, Dixon DL. Monocyte chemotactic protein (MCP)-1 (CCL2) and its receptor (CCR2) are elevated in chronic heart failure facilitating lung monocyte infiltration and differentiation which may contribute to lung fibrosis. Cytokine. 2023 Jan;161:156060. doi: 10.1016/j.cyto.2022.156060. Epub 2022 Oct 8. PMID: 36219898.
- Zhang K, Phan SH. Cytokines and pulmonary fibrosis. Biol Signals. 1996 Jul-Aug;5(4):232-9. doi: 10.1159/000109195. PMID: 8891199.
- Pulito-Cueto V, Remuzgo-Martínez S, Genre F, Atienza-Mateo B, Mora-Cuesta VM, Iturbe-Fernández D, Lera-Gómez L, Sebastián Mora-Gil M, Prieto-Peña D, Portilla V, Blanco R, Corrales A, Ocejo-Vinyals JG, Gualillo O, Ferraz-Amaro I, Cifrián JM, López-Mejías R, González-Gay MA. Elevated VCAM-1, MCP-1 and ADMA serum levels related to pulmonary fibrosis of interstitial lung disease associated with rheumatoid arthritis. Front Mol Biosci. 2022 Dec 19;9:1056121. doi: 10.3389/fmolb.2022.1056121. PMID: 36601584; PMCID: PMC9806218.
- Chen H, Chen H, Liang J, Gu X, Zhou J, Xie C, Lv X, Wang R, Li Q, Mao Z, Sun H, Zuo G, Miao D, Jin J. TGF-β1/IL-11/MEK/ERK signaling mediates senescence-associated pulmonary fibrosis in a stress-induced premature senescence model of Bmi-1 deficiency. Exp Mol Med. 2020 Jan;52(1):130-151. doi: 10.1038/s12276-019-0371-7. Epub 2020 Jan 21. PMID: 31959867; PMCID: PMC7000795.
- Inui N, Sakai S, Kitagawa M. Molecular Pathogenesis of Pulmonary Fibrosis, with Focus on Pathways Related to TGF-β and the Ubiquitin-Proteasome Pathway. Int J Mol Sci. 2021 Jun 5;22(11):6107. doi: 10.3390/ijms22116107. PMID: 34198949; PMCID: PMC8201174.
- 10 Peng L, Wen L, Shi QF, Gao F, Huang B, Meng J, Hu CP, Wang CM. Scutellarin ameliorates pulmonary fibrosis through inhibiting NF-κB/NLRP3-mediated epithelial-mesenchymal transition and inflammation. Cell Death Dis. 2020 Nov 13;11(11):978. doi: 10.1038/s41419-020-03178-2. PMID: 33188176; PMCID: PMC7666141.
- Racanelli AC, Kikkers SA, Choi AMK, Cloonan SM. Autophagy and inflammation in chronic respiratory disease. Autophagy. 2018;14(2):221-232. doi: 10.1080/15548627.2017.1389823. Epub 2018 Feb 8. PMID: 29130366; PMCID: PMC5902194.
- Cui H, Liu X, Zhang J, Zhang K, Yao D, Dong S, Feng S, Yang L, Li Y, Wang H, Huang J, Wang J. Rhodiola rosea L. Attenuates Cigarette Smoke and Lipopolysaccharide-Induced COPD in Rats via Inflammation Inhibition and Antioxidant and Antifibrosis Pathways. Evid Based Complement Alternat Med. 2021 Mar 2;2021:6103158. doi: 10.1155/2021/6103158. PMID: 33747104; PMCID: PMC7943302.
- Yang HZ, Wang JP, Mi S, Liu HZ, Cui B, Yan HM, Yan J, Li Z, Liu H, Hua F, Lu W, Hu ZW. TLR4 activity is required in the resolution of pulmonary inflammation and fibrosis after acute and chronic lung injury. Am J Pathol. 2012 Jan;180(1):275-92. doi: 10.1016/j.ajpath.2011.09.019. Epub 2011 Nov 7. PMID: 22062220.
- Liu S, Wu J, Chen P, Mohammed SAD, Zhang J, Liu S. TAK-242 Ameliorates Hepatic Fibrosis by Regulating the Liver-Gut Axis. Biomed Res Int. 2022 Aug 16;2022:4949148. doi: 10.1155/2022/4949148. PMID: 36017390; PMCID: PMC9398794.
- Liang GB, He ZH. Animal models of emphysema. Chin Med J (Engl). 2019 Oct 20;132(20):2465-2475. doi: 10.1097/CM9.0000000000000469. PMID: 31567388; PMCID: PMC6831071.
- Ghorani V, Boskabady MH, Khazdair MR, Kianmeher M. Experimental animal models for COPD: a methodological review. Tob Induc Dis. 2017 May 2;15:25. doi: 10.1186/s12971-017-0130-2. PMID: 28469539; PMCID: PMC5414171.
- Upadhyay P, Wu CW, Pham A, Zeki AA, Royer CM, Kodavanti UP, Takeuchi M, Bayram H, Pinkerton KE. Animal models and mechanisms of tobacco smoke-induced chronic obstructive pulmonary disease (COPD). J Toxicol Environ Health B Crit Rev. 2023 Jul 4;26(5):275-305. doi: 10.1080/10937404.2023.2208886. Epub 2023 May 14. PMID: 37183431; PMCID: PMC10718174.
- Sakornsakolpat P, Prokopenko D, Lamontagne M, Reeve NF, Guyatt AL, Jackson VE, Shrine N, Qiao D, Bartz et al; SpiroMeta Consortium; International COPD Genetics Consortium. Genetic landscape of chronic obstructive pulmonary disease identifies heterogeneous cell-type and phenotype associations. Nat Genet. 2019 Mar;51(3):494-505. doi: 10.1038/s41588-018-0342-2. Epub 2019 Feb 25. PMID: 30804561; PMCID: PMC6546635.
- Fathimath Muneesa M, Shaikh SB, Jeena TM, Bhandary YP. Inflammatory mediators in various molecular pathways involved in the development of pulmonary fibrosis. Int Immunopharmacol. 2021 Jul;96:107608. doi: 10.1016/j.intimp.2021.107608. Epub 2021 Apr 12. PMID: 33857801.
- Lee JW, Chun W, Lee HJ, Min JH, Kim SM, Seo JY, Ahn KS, Oh SR. The Role of Macrophages in the Development of Acute and Chronic Inflammatory Lung Diseases. Cells. 2021 Apr 14;10(4):897. doi: 10.3390/cells10040897. PMID: 33919784; PMCID: PMC8070705.
- Yi XM, Li M, Chen YD, Shu HB, Li S. Reciprocal regulation of IL-33 receptor-mediated inflammatory response and pulmonary fibrosis by TRAF6 and USP38. Proc Natl Acad Sci U S A. 2022 Mar 8;119(10):e2116279119. doi: 10.1073/pnas.2116279119. Epub 2022 Mar 1. PMID: 35238669; PMCID: PMC8917384.
- Wang Y, Sang X, Shao R, Qin H, Chen X, Xue Z, Li L, Wang Y, Zhu Y, Chang Y, Gao X, Zhang B, Zhang H, Yang J. Xuanfei Baidu Decoction protects against macrophages induced inflammation and pulmonary fibrosis via inhibiting IL-6/STAT3 signaling pathway. J Ethnopharmacol. 2022 Jan 30;283:114701. doi: 10.1016/j.jep.2021.114701. Epub 2021 Oct 1. PMID: 34606948; PMCID: PMC9715986.
- Lv Q, Wang J, Xu C, Huang X, Ruan Z, Dai Y. Pirfenidone alleviates pulmonary fibrosis in vitro and in vivo through regulating Wnt/GSK-3β/β-catenin and TGF-β1/Smad2/3 signaling pathways. Mol Med. 2020 May 24;26(1):49. doi: 10.1186/s10020-020-00173-3. PMID: 32448163; PMCID: PMC7245944.
- Zhao W, Wang L, Yang J, Chen X, Guo X, Xu K, Wang N, Zhao W, Xia C, Lian H, Rosas I, Yu G. Endothelial cell-derived MMP19 promotes pulmonary fibrosis by inducing E(nd)MT and monocyte infiltration. Cell Commun Signal. 2023 Mar 13;21(1):56. doi: 10.1186/s12964-023-01040-4. PMID: 36915092; PMCID: PMC10009991.
- Dehghanbanadaki H, Forouzanfar K, Kakaei A, Zeidi S, Salehi N, Arjmand B, Razi F, Hashemi E. The role of CDH2 and MCP-1 mRNAs of blood extracellular vesicles in predicting early-stage diabetic nephropathy. PLoS One. 2022 Apr 1;17(4):e0265619. doi: 10.1371/journal.pone.0265619. PMID: 35363774; PMCID: PMC8975111.
- Wonnacott A, Denby L, Coward RJM, Fraser DJ, Bowen T. MicroRNAs and their delivery in diabetic fibrosis. Adv Drug Deliv Rev. 2022 Mar;182:114045. doi: 10.1016/j.addr.2021.114045. Epub 2021 Nov 9. PMID: 34767865.
- Wang W, Zheng F, Zhang A. Arsenic-induced lung inflammation and fibrosis in a rat model: Contribution of the HMGB1/RAGE, PI3K/AKT, and TGF-β1/SMAD pathways. Toxicol Appl Pharmacol. 2021 Dec 1;432:115757. doi: 10.1016/j.taap.2021.115757. Epub 2021 Oct 19. PMID: 34673086.
- Qi W, Chen X, Polhill TS, Sumual S, Twigg S, Gilbert RE, Pollock CA. TGF-beta1 induces IL-8 and MCP-1 through a connective tissue growth factor-independent pathway. Am J Physiol Renal Physiol. 2006 Mar;290(3):F703-9. doi: 10.1152/ajprenal.00254.2005. Epub 2005 Oct 4. PMID: 16204411.
- Cho ML, Yoon BY, Ju JH, Jung YO, Jhun JY, Park MK, Park SH, Cho CS, Kim HY. Expression of CCR2A, an isoform of MCP-1 receptor, is increased by MCP-1, CD40 ligand and TGF-beta in fibroblast like synoviocytes of patients with RA. Exp Mol Med. 2007 Aug 31;39(4):499-507. doi: 10.1038/emm.2007.55. PMID: 17934338.
- Matsunaga N, Tsuchimori N, Matsumoto T, Ii M. TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol Pharmacol. 2011 Jan;79(1):34-41. doi: 10.1124/mol.110.068064. Epub 2010 Sep 29. PMID: 20881006.
- Liu W, Han X, Li Q, Sun L, Wang J. Iguratimod ameliorates bleomycin-induced pulmonary fibrosis by inhibiting the EMT process and NLRP3 inflammasome activation. Biomed Pharmacother. 2022 Sep;153:113460. doi: 10.1016/j.biopha.2022.113460. Epub 2022 Jul 25. PMID: 36076570.