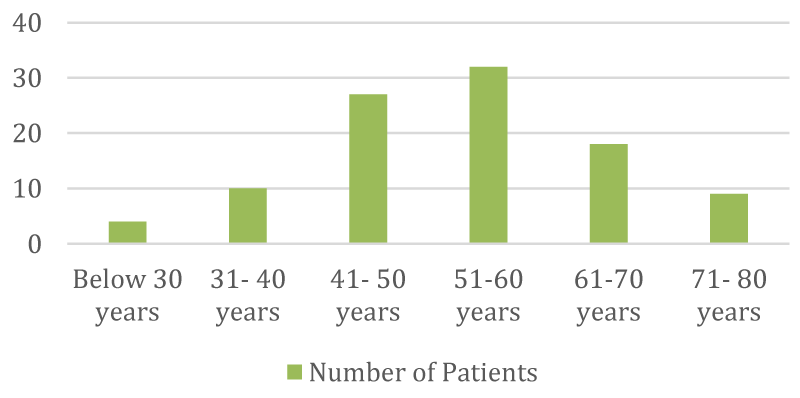More Information
Submitted: February 28, 2024 | Approved: April 01, 2024 | Published: April 02, 2024
How to cite this article: Hussaini SZK, Jilla S, Vishesh G, Pallavardhan P, Deekshita K, et al. A Prospective Observational Study on Neurotoxicity of Chemotherapy - A Critical Analysis. J Radiol Oncol. 2024; 8: 022-029.
DOI: 10.29328/journal.jro.1001061
Copyright License: © 2024 Hussaini SZK, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Cancer; Chemotherapy; Peripheral neuropathy; Paraesthesia; Neurotoxicity
A Prospective Observational Study on Neurotoxicity of Chemotherapy - A Critical Analysis
Syeda Zaineb Kubra Hussaini1*, Swapna Jilla2, Gumdal Vishesh3, Peddapalegani Pallavardhan4, Kalidindi Deekshita5, Kashadatla Rashmitha5, Kavuri Chaitanya5 and Kommu Kathyayani5
1Department of Pharmacy Practice, Malla Reddy Pharmacy College, Hyderabad, Telangana, India
2Radiation Oncologist, Malla Reddy Narayana Cancer Hospital and Research Institute, Hyderabad, Telangana, India
3Senior Consultant Medical Oncology, Malla Reddy Narayana Cancer Hospital and Research Institute Hyderabad, Telangana, India
4Ph.D., Research Scholar, Department of Statistics, Sri Venkateshwara University, Tirupathi, Andhra Pradesh, India
5Pharm. D (Doctor of Pharmacy), Malla Reddy Pharmacy College, Hyderabad, Telangana, India
*Address for Correspondence: Dr. Syeda Zaineb Kubra Hussaini, Department of Pharmacy Practice, Malla Reddy Pharmacy College, Hyderabad, Telangana, India
Background: Cancer treatment frequently depends on the intricate and potent effects that are acknowledged for their potential to save lives. Chemotherapy can have adverse effects on both the central and peripheral nervous systems, posing significant challenges.
Objective:
• To assess the causative agent, development, and timing of occurrence.
• To improve management of neurological complications.
• To discriminate the iatrogenic effects of cancer therapy and neurological progression.
Method: A prospective observational study was conducted in a hospital setting, focusing on the neurotoxic effects of chemotherapy in cancer patients over a span of six months. The research involved participants from both the oncology in-patient and daycare departments. After obtaining informed consent, individuals in the study population were interviewed to gather information about any neurological symptoms they encountered following their chemotherapy sessions.
Questionnaire and discussion: Within our study population, a predominant 67% comprised female patients, while male patients constituted 33%. Of the total participants, 66% reported experiencing neurological symptoms. Among these symptoms, the majority of patients encountered sensations such as tingling, numbness, and a burning sensation. Other reported symptoms included headaches, distal weakness, myalgia, seizures, and ataxia.
Discussion: In this current study, 66% of the study population encountered neurological side effects. Generally, the presence of comorbidities, vitamin deficiencies, and advanced age can significantly contribute to the development of peripheral neuropathy. Depending on the severity of neuropathy, recommendations for interventions include the prescription of vitamin supplements, calcium supplements, duloxetine, and pregabalin.
Conclusion: In this current study, 66% of the study population encountered neurological side effects. Generally, the presence of comorbidities, vitamin deficiencies, and advanced age can significantly contribute to the development of peripheral neuropathy. Depending on the severity of neuropathy, recommendations for interventions include the prescription of vitamin supplements, calcium supplements, duloxetine, and pregabalin.
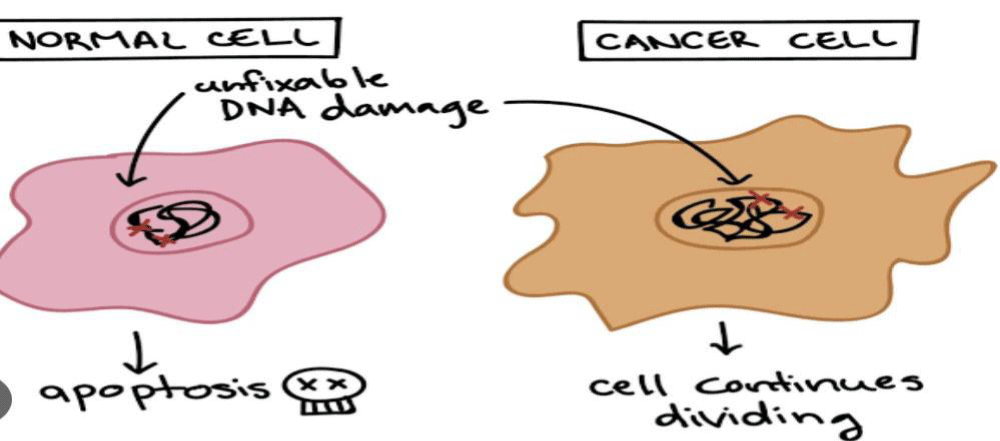
The neurotoxic effects associated with chemotherapy, affecting either the central or peripheral nervous system, impose a significant burden on patients and greatly diminish their quality of life. These neurotoxicities are often dose-limiting and can even necessitate the discontinuation of treatment, thereby compromising the effectiveness of cancer therapy. The review underscores the importance of early recognition of complications, facilitating prompt symptom management, adjustments in drug dosage, and differentiation between the adverse effects of cancer treatment and neurological deterioration due to the disease itself (Figure 1).
Figure 1: Cancer cell.
It elaborates on the separate manifestations of chemotherapy-induced side effects in the Central Nervous System and Peripheral Nervous System (CNS and PNS), with particular attention to the long-term cognitive decline and peripheral neuropathy, which exemplify the chronic neurotoxicity associated with chemotherapy [1,2].
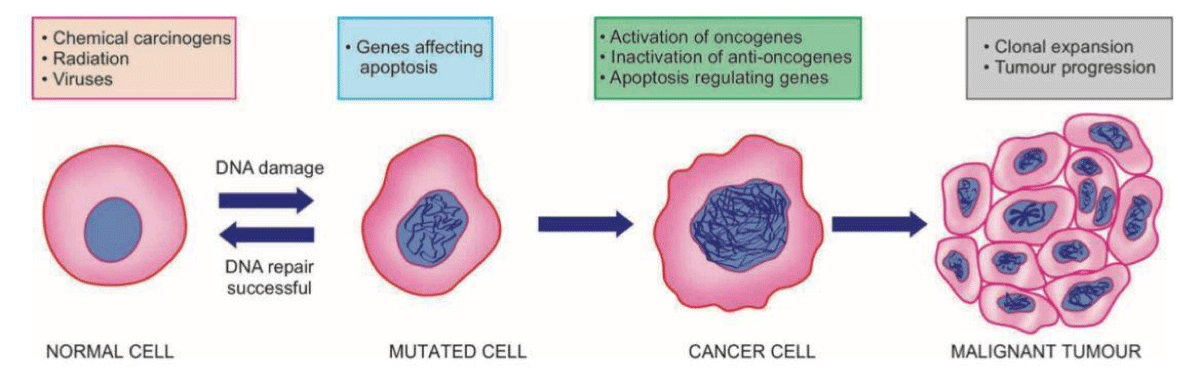
Furthermore, chemotherapy induces alterations in ion channels found in dorsal root ganglia and dorsal horn neurons, leading to subsequent modifications that give rise to neuropathic pain [3] (Figure 2).
Figure 2: Systemic illustration to show molecular basis of cancer.
Among the diverse neurological side effects, a subset of cancer survivors may experience cognitive impairment and mental depression, albeit typically in subtle forms. Various factors can either safeguard against cognitive impairment or elevate the risk of impaired cognitive function. These factors encompass the concurrent impact of cancer and its treatments such as medications, depression, and anxiety, as well as both the direct and indirect effects of chemotherapy, such as chemotherapy-induced anemia or menopause [4]. Despite concerted efforts to address the neurological side effects of chemotherapy in patients, and the ongoing advancements in managing these effects, challenges persist in the realm of neurological side effects [5].
The most prevalent complication affecting the peripheral nervous system in cancer therapy is Chemotherapy-Induced Peripheral Neuropathy (CIPN), and it can significantly impact the quality of life for patients [6-9]. Onset of Chemotherapy-Induced Peripheral Neuropathy (CIPN) symptoms may occur following the initial cycle of chemotherapy or, more commonly, with increasing frequency after subsequent cycles [8,10].
Chemotherapy-Induced Peripheral Neuropathy (CIPN) is primarily a sensory dysfunction, not a motor or autonomic dysfunction, which resolves after treatment discontinuation but may persist for years or be permanent [11]. Chemotherapy induces changes in ion channels of spinal ganglia and dorsal horn neurons that produce secondary changes that cause neuropathic pain [12]. Even with timely identification of neurotoxicity, the neurological repercussions of chemotherapy can endure, necessitating symptomatic intervention, such as addressing Chemotherapy-Induced Peripheral Neuropathy (CIPN) and chronic cognitive impairment [13].
Neurotoxicity may occur either independently or through a combination of mechanisms, including direct harm to neurons, changes in neuronal network dynamics, decreased neurogenesis and gliogenesis, overstimulation of supporting glial cells (such as in chemo brain), neuroinflammation and alterations in neuroendocrine function, heightened oxidative stress, and genetic mutations [14]. Chemotherapy-Induced Peripheral Neuropathy (CIPN) arises from chemotherapy’s inability to distinguish between cancerous and normal cells [15]. Neurotoxic chemotherapeutic agents should be closely monitored, and if deemed clinically appropriate (such as when patients exhibit signs of cancer regression), dose adjustments or combination therapies with less neurotoxic anticancer drugs may be considered. If these approaches prove ineffective, over-the-counter anti-neuropathic supplements like calcium or magnesium might be suggested [16]. Chemotherapy-induced peripheral neuropathy (CIPN) poses a significant challenge as a side effect of neurotoxic chemotherapy. Research into Chemotherapy-induced peripheral neuropathy (CIPN) risk factors has expanded in recent years, yet findings can be inconsistent or may only explore a narrow range of potential factors [17].
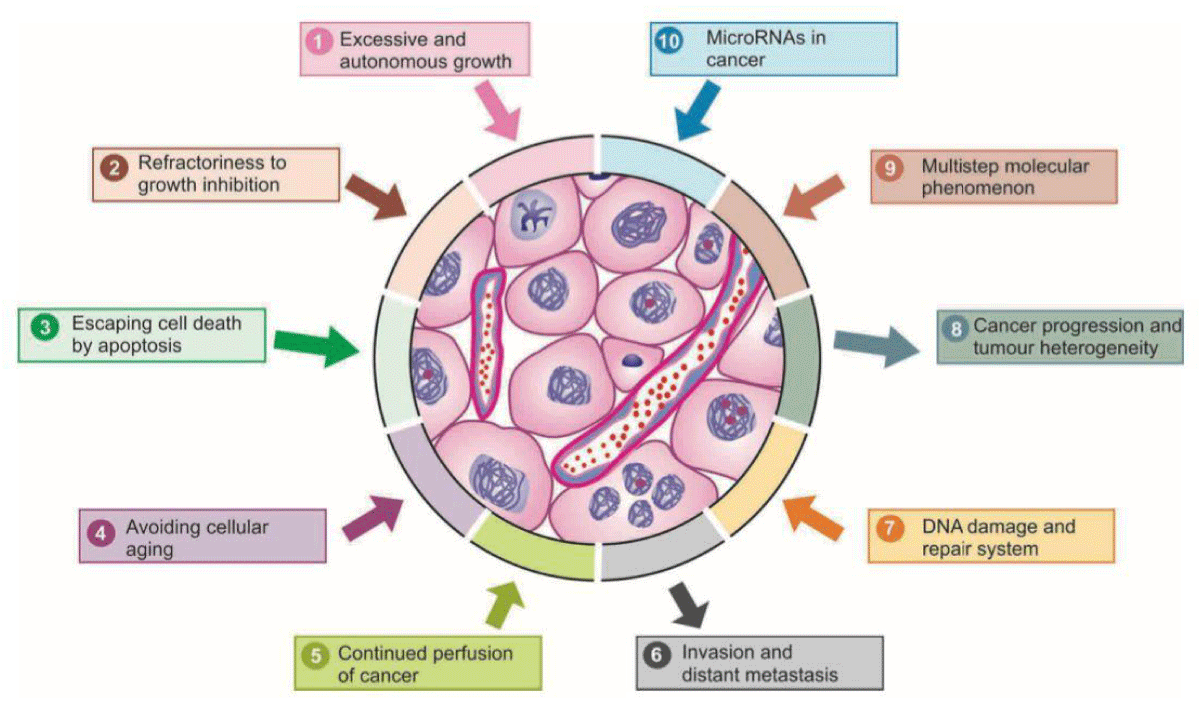
Enhancing our comprehension of the pathophysiology driving the onset of Chemotherapy-Induced Peripheral Neuropathy (CIPN), particularly the varied mechanisms across different chemotherapies, appears vital for devising forthcoming neuroprotective approaches [18]. Chemotherapy-Induced Peripheral Neuropathy (CIPN) stands out as a prevalent adverse effect associated with taxanes, often necessitating treatment adjustments or discontinuation due to its persistence. Motor neuropathy, typically mild, manifests as muscle weakness, resulting in compromised motor abilities such as fine motor movements, foot drop, or challenges with stair climbing [19]. The manifestations of Chemotherapy Induced Peripheral Neuropathy (CIPN) predominantly involve sensory symptoms such as pain, numbness, and tingling, commonly affecting the hands and feet [20]. The oncologist faces the challenge of effectively treating cancer while striving to minimize treatment toxicity to other organs. Both new and existing drugs exhibit potential in research aimed at mitigating Peripheral Nervous System (PNS) toxicity [21] (Figure 3).
Figure 3: Schematic representation of major properties of cancer in terms of molecular carcinogens.
Chemotherapy-Induced Peripheral Neuropathy(CIPN) represents a prevalent and significant issue, posing diagnostic and therapeutic hurdles, and importantly, significantly impacting the patient’s quality of life and the management of their cancer treatment [22]. Chemotherapy, particularly cisplatin therapy, has the potential to induce central nervous system (CNS) toxicity, leading to a decline in cognitive function [23]. Systemic therapy utilized in oncology can induce various adverse effects on the nervous system. Neurotoxicity often prompts dose adjustments or treatment cessation and, in severe cases, may pose a direct threat to life [24]. The growing population of cancer survivors, coupled with advancements in the efficacy of chemotherapy drugs and a heightened focus on patient-centered outcomes, is driving efforts to reduce the occurrence of Chemotherapy-Induced Peripheral Neuropathy (CIPN) [25]. Preemptively treating with suitable agents to diminish the frequency or intensity of Chemotherapy-Induced Peripheral Neuropathy (CIPN) is an ideal approach. While numerous drugs have undergone specific testing for this purpose, initial promising results have not translated into current usage or recommendations. According to the clinical practice guidelines issued by the American Society of Clinical Oncology (ASCO), the use of agents to prevent Chemotherapy-Induced Peripheral Neuropathy (CIPN) is not recommended. However, for the treatment of established Chemotherapy-induced peripheral neuropathy (CIPNs), American Society of Clinical Oncology (ASCO) guidelines moderately recommend duloxetine [26].
Managing non-neuropathic and neuropathic pain differs fundamentally: pharmacotherapy is typically employed for neuropathic pain, whereas non-neuropathic pain can often be addressed through physical therapy or behavioral training [27]. To mitigate the risk of undesirable interactions with chemotherapy drugs, non-enzyme-inducing antiepileptic drugs are favored [28]. Peripheral neuropathy represents a prevalent chronic complication of cancer and its treatment. It can arise from a range of chemotherapeutic agents, including Taxanes (such as paclitaxel and docetaxel), vinca alkaloids (like vincristine, vinorelbine, and vinblastine), platinum analogues (including cisplatin, carboplatin, and oxaliplatin), bortezomib, thalidomide, and other medications [29-31]. All vinca alkaloids exhibit significant neuropathic effects and can decrease gastrointestinal transit time. Among them, vincristine and vindesine demonstrate the most pronounced severity, while vinblastine shows lesser severity, and vinorelbine the least. The severity is dose-dependent in patients receiving vincristine treatment, with higher occurrences and severity observed in those administered doses exceeding 2 mg in total [32,33]. Moreover, certain chemotherapy drugs that readily penetrate the blood-brain barrier (e.g., 5-fluorouracil and methotrexate) can directly or indirectly impact brain functions, leading to issues such as memory impairment and depression [18,34].
Encephalopathy stands out as the predominant Central Nervous System (CNS) toxicity associated with chemotherapy, often manifesting as altered consciousness, seizures, neurobehavioral changes, or focal neurological deficits [35-37]. Movement disorders, extrapyramidal syndrome, ataxia, myoclonus, and complex partial status epilepticus are among the observable symptoms [38,39].
Objectives
To evaluate the responsible factor, progression, and timing of onset, aiming to enhance the management of neurological complications arising from chemotherapy. The goal is to distinguish between the iatrogenic effects of cancer therapy and neurological progression attributed to the cancer itself.
Study design
A prospective observational study was conducted in a hospital setting to examine chemotherapy-induced neurotoxicity among cancer patients over a duration of six months. The research was carried out in the Oncology Patient and Day Care Department at Malla Reddy Cancer Hospital and Research Institute in Hyderabad, India.
Study period: Six Months
Sample size: 100 Patients
Study criteria
Inclusion criteria: Patients above 5 years of age.
Patient presenting with chemotherapy induced neurological side effects.
Patients with risk factors such as pre-existing neuropathy due to DM, Alcohol, folate/vitamin B12 deficiency.
Patients who are willing to give informed consent.
Presence of severe concurrent diseases (Cardio-respiratory, renal, hepatic, neurological disorders etc).
Exclusion criteria: Patient less than 5 years of age.
Pregnant and lactating women.
Patient not expected to cooperate and comply with the treatment.
The Patient Neurotoxicity Questionnaire (PNQ) serves as a diagnostic tool for individuals experiencing Chemotherapy-Induced Peripheral Neuropathy (CIPN). An increasingly utilized method in clinical practice, the Peripheral Neurotoxicity Questionnaire (PNQ) aligns with established criteria and has demonstrated significantly positive results. Developed by Bionumik Pharmaceuticals, Inc., the Peripheral Neurotoxicity Questionnaire (PNQ) is a straightforward, self-administered assessment tool incorporating data from the Food and Drug Administration (FDA). It comprises specific questions designed to extract quantifiable and essential diagnostic information directly from Chemotherapy-Induced Peripheral Neuropathy (CIPN) patients, addressing both the severity and incidence of subjective Chemotherapy-Induced Peripheral Neuropathy (CIPN) symptoms.
The creation of the Patient Neurotoxicity Questionnaire (PNQ) was driven by the limitations of other questionnaires in reliably assessing and quantifying Chemotherapy-Induced Peripheral Neuropathy(CIPN). Despite frequent reporting of Chemotherapy-Induced Peripheral Neuropathy (CIPN) in various studies, information regarding its frequency, prevalence, onset, duration, regression, or persistence in patients undergoing chemotherapy remains elusive. Moreover, clinical studies seldom report the number of patients experiencing treatment delays or modifications due to Chemotherapy-Induced Peripheral Neuropathy (CIPN). Recent studies focused on Chemotherapy-Induced Peripheral Neuropathy (CIPN) symptomatology, comparing patient-centered reports on the severity and frequency of Chemotherapy-Induced Peripheral Neuropathy (CIPN) among individuals undergoing treatment.
Dietary recommendations:
1) Choose a mostly plant-based diet high in fruits, vegetables, and whole grains.
2) Limit red and processed meats.
3) Rinse vegetables and fruits thoroughly before eating them.
4) Eat fully cooked foods.
5) Avoid spicy foods or foods with strong odors.
6) Try to eat when you are feeling the best, no matter what time of day.
7) Eat small meals or nutritious snacks throughout the day, rather than three larger meals.
8) Include high protein, high-calorie diets and plenty of fluids.
9) A combination of a healthy diet and regular exercise can help to maintain a healthy weight.
10) Avoid smoking or exposure to tobacco smoke.
➢ Classification of patients according to age distribution
| Age | No | % |
| Below 30 years | 4 | 4% |
| 31 - 40 yrs | 10 | 10% |
| 41 - 50 yrs | 27 | 27% |
| 51 - 60 yrs | 32 | 32% |
| 61 - 70 yrs | 18 | 18% |
| 71 - 80 yrs | 9 | 9% |
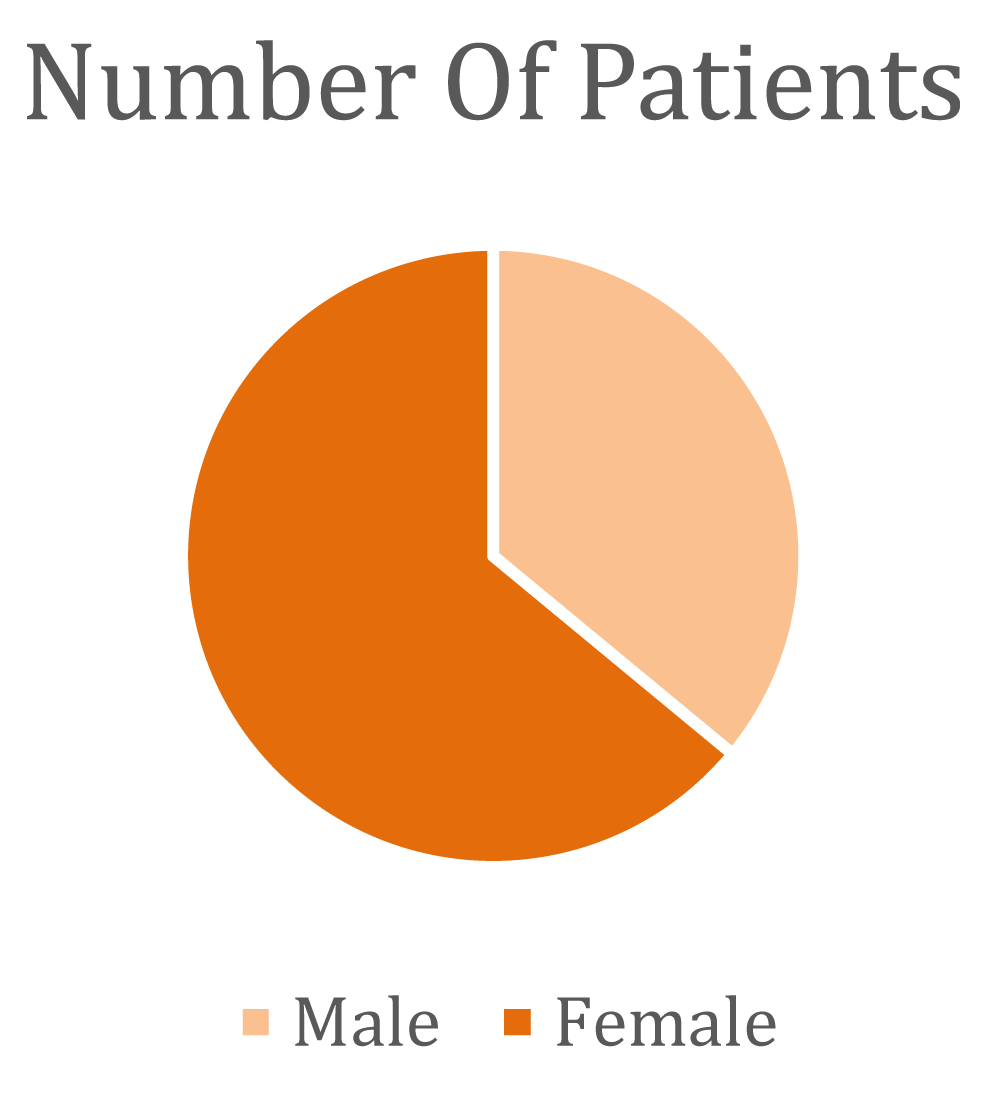
➢ Categorization of patients according to gender distribution
| Sex | No | % |
| Male | 36 | 36% |
| Female | 64 | 64% |
➢ Arrangement of patients according to their diagnosed conditions
| Type of Cancer | No. | % |
| Buccal Mucosa | 3 | 3% |
| Ca. Breast | 27 | 27% |
| Ca. Breast with Brain & Lungmets | 1 | 1% |
| Ca. Cervix | 10 | 10% |
| Ca. Ear | 1 | 1% |
| Ca. Esophagus | 1 | 1% |
| Ca. Gallbladder | 1 | 1% |
| Ca. Gastro Esophageal | 1 | 1% |
| Ca. Hypopharynx | 1 | 1% |
| Ca. Lung | 8 | 8% |
| Ca. Mid Oesophagus | 2 | 2% |
| Ca. Oesophagus | 3 | 3% |
| Ca. Ovary | 8 | 8% |
| Ca. Pancreas | 2 | 2% |
| Ca. Postcricoid | 1 | 1% |
| Ca. Rectum | 3 | 3% |
| Ca. Renal | 1 | 1% |
| Ca. Stomach | 4 | 4% |
| Ca. Stomach with GE Extension | 1 | 1% |
| Ca. Tongue | 3 | 3% |
| Ca. Vulva with inguinal & iliac node mets | 1 | 1% |
| Dual Malignancy | 1 | 1% |
| Hodgkins Lymphoma | 8 | 8% |
| Non Hodgkins Lymphoma | 2 | 2% |
| Osteosarcoma of knee joint | 1 | 1% |
| Primary Bone Lymphoma | 1 | 1% |
| Primary peritoneal Carcinoma | 1 | 1% |
| Pseudomyxoma Peritonei | 1 | 1% |
| Recurrent Soft Tissue Sarcoma of left thigh | 1 | 1% |
| Thymic Carcinoma | 1 | 1% |
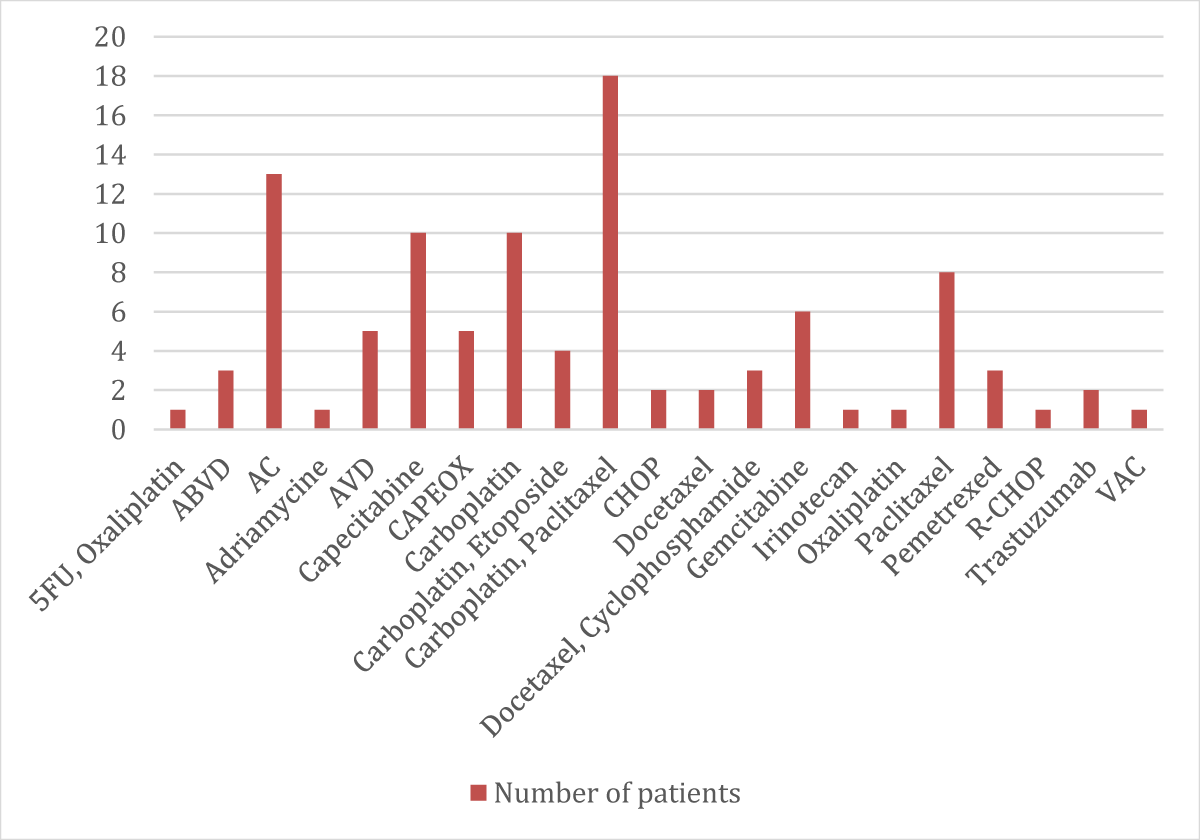
➢ Patients categorized according to their chemotherapy regimen distribution
| Chemotherapic Regimen | No. | % |
| 5FU, Oxaliplatin | 1 | 1% |
| ABVD | 3 | 3% |
| AC | 13 | 13% |
| Adriamycine | 1 | 1% |
| AVD | 5 | 5% |
| Capecitabine | 10 | 10% |
| CAPEOX | 5 | 5% |
| Carboplatin | 10 | 10% |
| Carboplatin, Etoposide | 4 | 4% |
| Carboplatin, Paclitaxel | 18 | 18% |
| CHOP | 2 | 2% |
| Docetaxel | 2 | 2% |
| Docetaxel, Cyclophosphamide | 3 | 3% |
| Gemcitabine | 6 | 6% |
| Irinotecan | 1 | 1% |
| Oxaliplatin | 1 | 1% |
| Paclitaxel | 8 | 8% |
| Pemetrexed | 3 | 3% |
| R-CHOP | 1 | 1% |
| Trastuzumab | 2 | 2% |
| VAC | 1 | 1% |
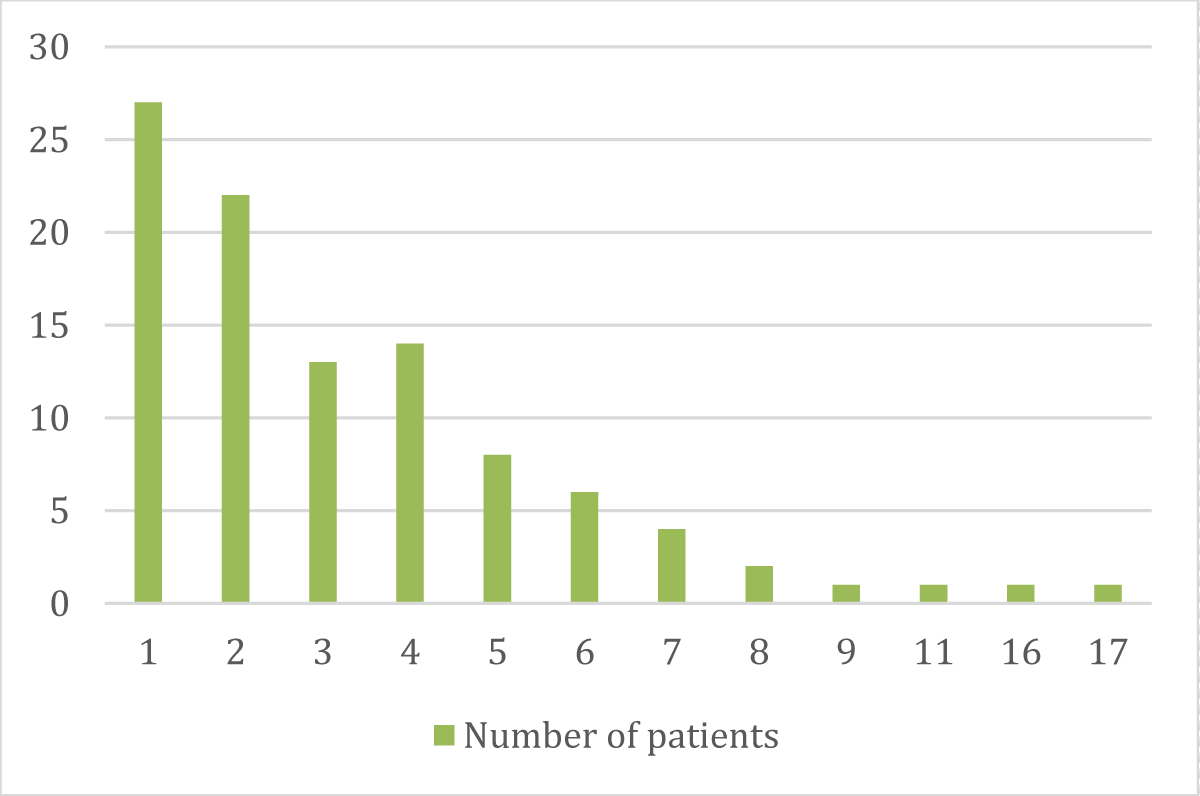
➢ Patients distributed according to the number of chemotherapy cycles they undergo
| No. of Cycles | No. | % |
| 1 | 27 | 27% |
| 2 | 22 | 22% |
| 3 | 13 | 13% |
| 4 | 14 | 14% |
| 5 | 8 | 8% |
| 6 | 6 | 6% |
| 7 | 4 | 4% |
| 8 | 2 | 2% |
| 9 | 1 | 1% |
| 11 | 1 | 1% |
| 16 | 1 | 1% |
| 17 | 1 | 1% |
➢ Patients categorized according to the dosage of cytotoxic drugs administered
| Cytotoxic Drug & Dose | No. | % |
| 5FU 700 mg, Oxaliplatin 150 mg | 1 | 1% |
| Adriamycin 100 mg, Cyclophosphamide 1000 mg | 3 | 3% |
| Adriamycin 120 mg, Cyclophosphamide 1200 mg | 1 | 1% |
| Adriamycin 25 mg, Vinblastine 6 mg, Bleomycin 10iu, Dacarbazine 375 mg | 1 | 1% |
| Adriamycin 25 mg, Vinblastine 6 mg, Dacarbazine 600 mg | 1 | 1% |
| Adriamycin 25 mg,Vinblastine 6 mg, Bleomycin 9 mg, Dacarbazine 350 mg | 1 | 1% |
| Adriamycin 35 mg, Vinblastine 9 mg, Dacarbazine 530 mg | 1 | 1% |
| Adriamycin 38 mg,Vinblastine 9 mg, Bleomycin 15 mg, Dacarbazine 560 mg | 1 | 1% |
| Adriamycin 40 mg, Vinblastine 10 mg, Dacarbazine 570 mg | 1 | 1% |
| Adriamycin 80 mg, Cyclophosphamide 800 mg | 1 | 1% |
| Adriamycin 90 mg, Cyclophosphamide 900 mg | 4 | 4% |
| Adriamycin-100 mg, Cyclophosphamide-1000 mg | 1 | 1% |
| Adriamycine 80 mg | 1 | 1% |
| Adriamycine 80 mg, Cyclophosphamide 500 mg | 1 | 1% |
| Adriamycine 90 mg, Cyclophosphamide 900 mg | 1 | 1% |
| Adriamycine 95 mg, Cyclophosphamide 950 mg | 1 | 1% |
| Capacitabine 500 mg, Oxaliplatin 200 mg | 5 | 5% |
| Capecitabine 500 mg | 10 | 10% |
| Carboplatin 150 mg | 1 | 1% |
| Carboplatin 150 mg, Paclitaxel 150 mg | 1 | 1% |
| Carboplatin 300 mg | 7 | 7% |
| Carboplatin 300 mg, Etoposide 100 mg | 1 | 1% |
| Carboplatin 300 mg, Paclitaxel 200 mg | 1 | 1% |
| Carboplatin 300 mg, Paclitaxel 230 mg | 1 | 1% |
| Carboplatin 335 mg, Paclitaxel 260 mg | 1 | 1% |
| Carboplatin 450 mg | 2 | 2% |
| Carboplatin 450 mg, Paclitaxel 220 mg | 14 | 14% |
| Cyclophasphamyde 500 mg, Adriaamycin 20 mg, Vincristine 1 mg | 2 | 2% |
| Docetaxel 100 mg | 1 | 1% |
| Docetaxel-100 mg, Cyclophosphamide-700 mg | 1 | 1% |
| Docetaxel-100 mg, Cyclophosphamide-800 mg | 1 | 1% |
| Docetaxel-120 mg | 1 | 1% |
| Docetaxel-120 mg, Cyclophosphamide-1000 mg | 1 | 1% |
| Etopaside 100 mg, Carboplatin 300 mg | 1 | 1% |
| Etopaside 130 mg | 1 | 1% |
| Etopaside 500 mg, Carboplatin 450 mg | 1 | 1% |
| Gemcitabine 1000 mg | 3 | 3% |
| Gemcitabine 1400 mg | 3 | 3% |
| Irinotecan 100 mg | 1 | 1% |
| Oxaliplatin 200 mg | 1 | 1% |
| Paclitaxel 150 mg | 1 | 1% |
| Paclitaxel 200 mg | 3 | 3% |
| Paclitaxel 220 mg | 1 | 1% |
| Paclitaxel 240 mg | 1 | 1% |
| Paclitaxel 250 mg | 1 | 1% |
| Paclitaxel 260 mg | 1 | 1% |
| Pemetrexed 400 mg | 1 | 1% |
| Pemetrexed 500 mg | 2 | 2% |
| Rituximab 600 mg | 1 | 1% |
| Trastuzumab 390 mg | 2 | 2% |
| Vinblastine 10 mg, Bleomycin 15iu, Dacarbazine 560 mg | 1 | 1% |
| Vinblastine 10 mg, Dacarbazine 500 mg | 1 | 1% |
| Vincristine 2 mg, Actinomycin 2 mg, Cyclophasphamyde + Mesna | 1 | 1% |
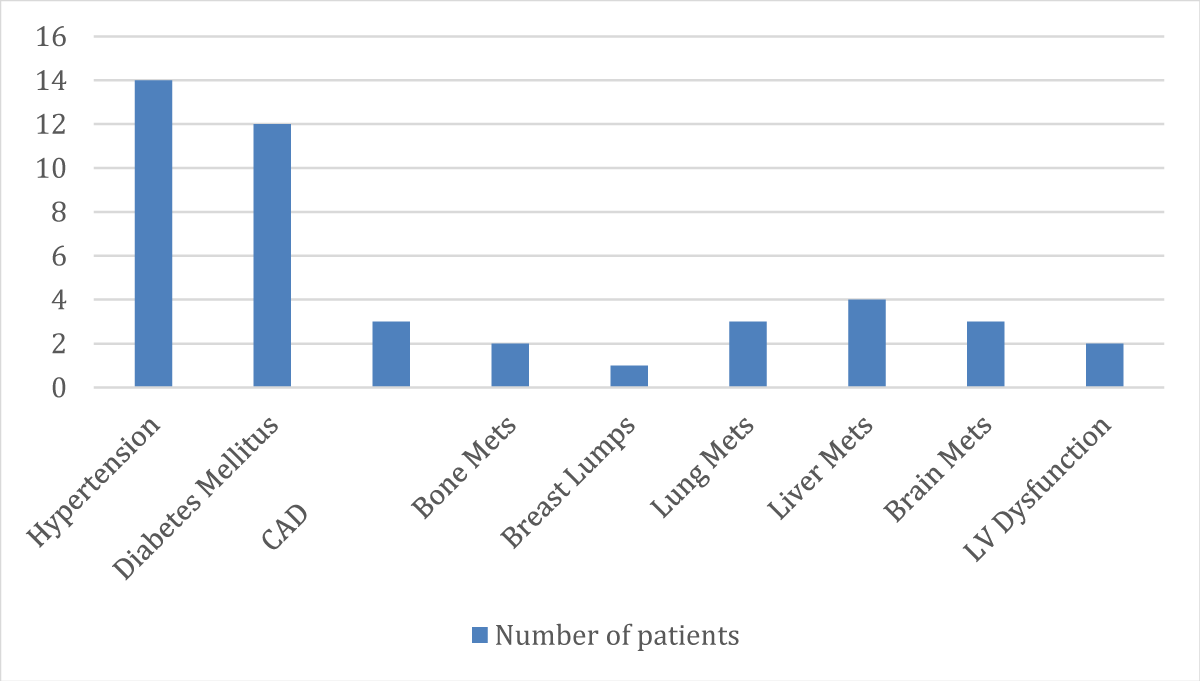
➢ Patients classified according to their comorbidity distribution
| S.No. | Past Medical History | No. of Patients |
| 1. | Hypertension | 14 |
| 2. | Diabetes Mellitus | 12 |
| 3. | CAD | 03 |
| 4. | Bone Mets | 02 |
| 5. | Breast Lumps | 01 |
| 6. | Lung Mets | 03 |
| 7. | Liver Mets | 04 |
| 8. | Brain Mets | 03 |
| 9. | LV Dysfunction | 02 |
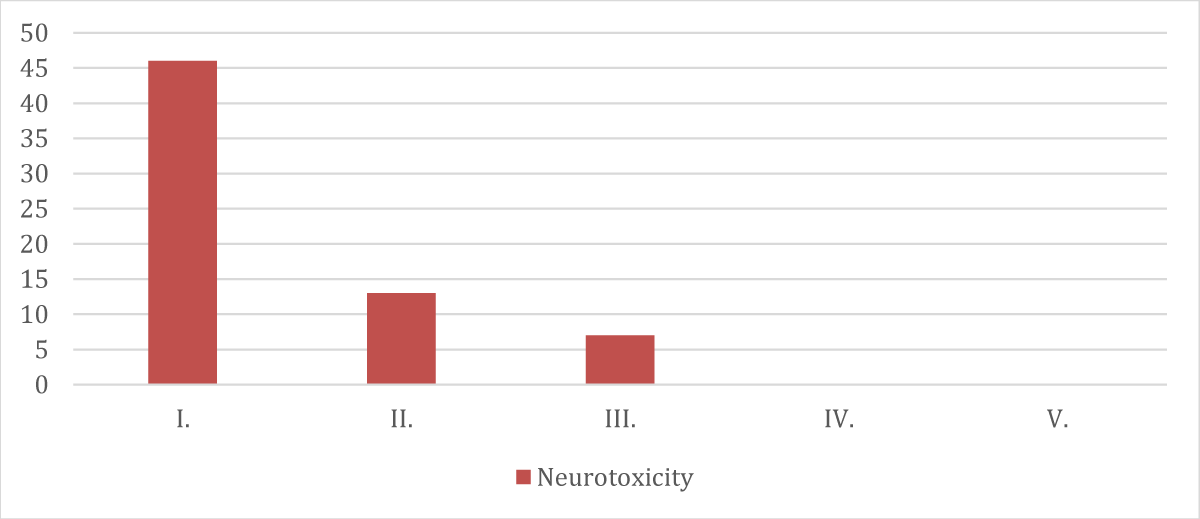
➢ Patients categorized according to neurotoxicity distribution
| Grade | Neurotoxicity (Peripheral Neuropathy) |
| I. | 46 |
| II. | 13 |
| III. | 7 |
| IV. | 0 |
| V. | 0 |
Chemotherapy can have severe impacts on both the Central nervous system and Peripheral nervous systems (CNS and PNS) In the Peripheral Nervous System (PNS), Chemotherapy-Induced Peripheral Neuropathy (CIPN) and myopathy stand out as significant complications. In cases of severe Chemotherapy-Induced Peripheral Neuropathy (CIPN), chemotherapy dosage may be reduced, administration delayed, or treatment interrupted. Treatment options for Chemotherapy-Induced Peripheral Neuropathy (CIPN) are limited, and a meta-analysis identified duloxetine as the preferred symptomatic remedy. The study included 100 participants, with 37% being male and 63% female.
- A total study involves 100 patients, with 37% being male and 63% female.
- The majority of individuals undergoing chemotherapy fall within the age range of 46 to 55 years.
- Among the 100 patients, 27 were diagnosed with breast cancer, 22 with gastrointestinal diseases, and 14 with gynaecological tumours.
- 44 patients out of 100 are viewed as comorbid (Patients with Hypertension-14 and patients with Diabetes Mellitus-12)
- 66 patients out of 100 experienced neurological side effects.
- 58 patients experienced shivering, deadness and consuming sensation in their grasp and feet in the wake of taking chemotherapy.
- 44 out of 100 patients experienced Neurotoxicity with fringe neuropathy, 14 with Paraesthesia, 3 with seizures, 19 with Distal Shortcoming, 10 with Agony, 9 with Myalgia, 3 with Ataxia and 2 With Obscured Vision.
- The largest number of patients has taken carboplatin (n = 32) and followed by paclitaxel (n = 30)
Patients receiving carboplatin, paclitaxel, vincristine, vinblastine, or capecitabine have reported peripheral neuropathy. Those taking carboplatin and paclitaxel have experienced paraesthesia, while individuals on Adriamycin and Cyclophosphamide have encountered seizures. Docetaxel and gemcitabine users have faced distal weakness, vincristine and carboplatin recipients have reported pain, and those on capecitabine, carboplatin, and paclitaxel have described dizziness. Patients taking cyclophosphamide and carboplatin have reported headaches.
Chemotherapy may have detrimental effects the Central Nervous System or Peripheral Nervous System (CNS and PNS). Neurotoxicity can manifest in various ways, such as peripheral neuropathy (damage to nerves outside the brain and spinal cord), cognitive impairement (often referred to as “chemo brain”), and other neurological symptoms. The main objective of the study was to investigate neurotoxicity in patients undergoing chemotherapy. Chemotherapy can have detrimental effects on the nervous system, and factors such as advanced age, existing health conditions, and nutritional deficiencies are often linked to an increased risk of developing peripheral neuropathy. In this study, 66% of the participants reported neurological symptoms such as numbness, tingling, burning sensations in their arms and legs, seizures, myalgia, etc. Among them, 44 patients experienced peripheral neuropathy, and 14 suffered from paraesthesia. Additionally, chemotherapy-induced pain was observed in 19 patients.
- Ganz PA, Dougherty PM. Painful Hands and Feet After Cancer Treatment: Inflammation Affecting the Mind-Body Connection. J Clin Oncol. 2016 Mar 1;34(7):649-52. doi: 10.1200/JCO.2015.64.7479. Epub 2015 Dec 23. PMID: 26700128. An updated expert opinion on recent advances regarding chemotherapy –related neurotoxicity, underscoring the important role of inflammation in cognitive dysfunction, and peripheral neuropathy, both linked to neuroinflammation as well as genetic susceptibility to persistent inflammation andbehavioral symptoms. The authors also highlight that the symptoms of chemotherapy neurotoxicity can now be measured with a wide variety of well-validated self-report tools i.e. patient-reported outcomes.
- Nudelman KN, McDonald BC, Wang Y, Smith DJ, West JD, O'Neill DP, Zanville NR, Champion VL, Schneider BP, Saykin AJ. Cerebral Perfusion and Gray Matter Changes Associated with Chemotherapy-Induced Peripheral Neuropathy. J Clin Oncol. 2016 Mar 1;34(7):677-83. doi: 10.1200/JCO.2015.62.1276. Epub 2015 Nov 2. PMID: 26527786; PMCID: PMC4822503. Longitudinal relationship between chemotherapy-induced peripheral neuropathy (CIPN) symptoms and brain perfusion changes in patients with breast cancer. CIPN symptoms were positively associated with cerebral perfusion in the right superior frontal gyrus and cingulate gyrus, regions associated with pain processing.
- Jaggi AS, Singh N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology. 2012 Jan 27;291(1-3):1-9. doi: 10.1016/j.tox.2011.10.019. Epub 2011 Nov 3. PMID: 22079234.
- Jansen C, Miaskowski C, Dodd M, Dowling G, Kramer J. Potential mechanisms for chemotherapy-induced impairments in cognitive function. Oncol Nurs Forum. 2005 Nov 3;32(6):1151-63. doi: 10.1188/05.ONF.1151-1163. PMID: 16270111.
- Verstappen CC, Heimans JJ, Hoekman K, Postma TJ. Neurotoxic complications of chemotherapy in patients with cancer: clinical signs and optimal management. Drugs. 2003;63(15):1549-63. doi: 10.2165/00003495-200363150-00003. PMID: 12887262.
- Park SB, Goldstein D, Krishnan AV, Lin CS, Friedlander ML, Cassidy J, Koltzenburg M, Kiernan MC. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013 Nov-Dec;63(6):419-37. doi: 10.3322/caac.21204. PMID: 24590861.
- Rivera E, Cianfrocca M. Overview of neuropathy associated with taxanes for the treatment of metastatic breast cancer. Cancer Chemother Pharmacol. 2015 Apr;75(4):659-70. doi: 10.1007/s00280-014-2607-5. Epub 2015 Jan 18. PMID: 25596818; PMCID: PMC4365177.
- Eckhoff L, Knoop AS, Jensen MB, Ejlertsen B, Ewertz M. Risk of docetaxel-induced peripheral neuropathy among 1,725 Danish patients with early stage breast cancer. Breast Cancer Res Treat. 2013 Nov;142(1):109-18. doi: 10.1007/s10549-013-2728-2. Epub 2013 Oct 17. PMID: 24132874.
- Brewer JR, Morrison G, Dolan ME, Fleming GF. Chemotherapy-induced peripheral neuropathy: Current status and progress. Gynecol Oncol. 2016 Jan;140(1):176-83. doi: 10.1016/j.ygyno.2015.11.011. Epub 2015 Nov 7. PMID: 26556766; PMCID: PMC4698212.
- Grisold W, Cavaletti G, Windebank AJ. Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro Oncol. 2012 Sep;14 Suppl 4(Suppl 4):iv45-54. doi: 10.1093/neuonc/nos203. PMID: 23095830; PMCID: PMC3480245.
- Zhang S. Chemotherapy-induced peripheral neuropathy and rehabilitation: A review. Semin Oncol. 2021 Jun;48(3):193-207. doi: 10.1053/j.seminoncol.2021.09.004. Epub 2021 Sep 22. PMID: 34607709.
- Lee GD, Longo DL, Wang Y, Rifkind JM, Abdul-Raman L, Mamczarz JA, Duffy KB, Spangler EL, Taub DD, Mattson MP, Ingram DK. Transient improvement in cognitive function and synaptic plasticity in rats following cancer chemotherapy. Clin Cancer Res. 2006 Jan 1;12(1):198-205. doi: 10.1158/1078-0432.CCR-05-1286. PMID: 16397043.
- Taillibert S, Le Rhun E, Chamberlain MC. Chemotherapy-Related Neurotoxicity. Curr Neurol Neurosci Rep. 2016 Sep;16(9):81. doi: 10.1007/s11910-016-0686-x. PMID: 27443648.
- Was H, Borkowska A, Bagues A, Tu L, Liu JYH, Lu Z, Rudd JA, Nurgali K, Abalo R. Mechanisms of Chemotherapy-Induced Neurotoxicity. Front Pharmacol. 2022 Mar 28;13:750507. doi: 10.3389/fphar.2022.750507. PMID: 35418856; PMCID: PMC8996259.
- Beijers AJ, Jongen JL, Vreugdenhil G. Chemotherapy-induced neurotoxicity: the value of neuroprotective strategies. Neth J Med. 2012 Jan;70(1):18-25. PMID: 22271810.
- Banach M, Juranek JK, Zygulska AL. Chemotherapy-induced neuropathies—a growing problem for patients and health care providers. Brain Behav. 2017; 7. doi: 10.1002/BRB3.558.
- Molassiotis A, Cheng HL, Leung KT, Li YC, Wong KH, Au JSK, Sundar R, Chan A, Ng TR, Suen LKP, Chan CW, Yorke J, Lopez V. Risk factors for chemotherapy-induced peripheral neuropathy in patients receiving taxane- and platinum-based chemotherapy. Brain Behav. 2019 Jun;9(6):e01312. doi: 10.1002/brb3.1312. Epub 2019 May 7. PMID: 31063261; PMCID: PMC6576180.
- Park SB, Goldstein D, Krishnan AV, Lin CS, Friedlander ML, Cassidy J, Koltzenburg M, Kiernan MC. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013 Nov-Dec;63(6):419-37. doi: 10.3322/caac.21204. PMID: 24590861.
- Catalano M, Ramello M, Conca R, Aprile G, Petrioli R, Roviello G. Risk Factors for Nab-Paclitaxel and Gemcitabine-Induced Peripheral Neuropathy in Patients with Pancreatic Cancer. Oncology. 2022;100(7):384-391. doi: 10.1159/000524868. Epub 2022 May 12. PMID: 35551139.
- Ewertz M, Qvortrup C, Eckhoff L. Chemotherapy-induced peripheral neuropathy in patients treated with taxanes and platinum derivatives. Acta Oncol. 2015 May;54(5):587-91. doi: 10.3109/0284186X.2014.995775. Epub 2015 Mar 9. PMID: 25751757.
- Gutiérrez-Gutiérrez G, Sereno M, Miralles A, Casado-Sáenz E, Gutiérrez-Rivas E. Chemotherapy-induced peripheral neuropathy: clinical features, diagnosis, prevention and treatment strategies. Clin Transl Oncol. 2010 Feb;12(2):81-91. doi: 10.1007/S12094-010-0474-z. PMID: 20156778.
- Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol. 2006 Feb;33(1):15-49. doi: 10.1053/j.seminoncol.2005.12.010. PMID: 16473643.
- Troy L. Cisplatin-Based Therapy: a Neurological and Neuropsychological Review. Psychooncology. 2000; 9: 29-39.
- Gorzelak-Magiera A, Bobola A, Robek A, Krzystanek E, Gisterek I. Selected neurological complications of oncological treatment — literature overview. Oncology in Clinical Practice. 2022. doi: 10.5603/OCP.2022.0032.
- Argyriou AA, Bruna J, Genazzani AA, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity: management informed by pharmacogenetics. Nat Rev Neurol. 2017 Aug;13(8):492-504. doi: 10.1038/nrneurol.2017.88. Epub 2017 Jun 30. PMID: 28664909.
- Zajączkowska R, Kocot-Kępska M, Leppert W, Wrzosek A, Mika J, Wordliczek J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int J Mol Sci. 2019 Mar 22;20(6):1451. doi: 10.3390/ijms20061451. PMID: 30909387; PMCID: PMC6471666.
- Geber C, Breimhorst M, Burbach B, Egenolf C, Baier B, Fechir M, Koerber J, Treede RD, Vogt T, Birklein F. Pain in chemotherapy-induced neuropathy--more than neuropathic? Pain. 2013 Dec;154(12):2877-2887. doi: 10.1016/j.pain.2013.08.028. Epub 2013 Aug 30. PMID: 23999056.
- Grewal J, Grewal HK, Forman AD. Seizures and epilepsy in cancer: etiologies, evaluation, and management. Curr Oncol Rep. 2008 Jan;10(1):63-71. doi: 10.1007/s11912-008-0010-2. PMID: 18366962.
- Lee JJ, Swain SM. Peripheral neuropathy induced by microtubule-stabilizing agents. J Clin Oncol. 2006 Apr 1;24(10):1633-42. doi: 10.1200/JCO.2005.04.0543. PMID: 16575015.
- Kish JA, Ensley JF, Jacobs J, Weaver A, Cummings G, Al-Sarraf M. A randomized trial of cisplatin (CACP) + 5-fluorouracil (5-FU) infusion and CACP + 5-FU bolus for recurrent and advanced squamous cell carcinoma of the head and neck. Cancer. 1985 Dec 15;56(12):2740-4. doi: 10.1002/1097-0142(19851215)56:12<2740::aid-cncr2820561203>3.0.co;2-y. PMID: 3902199.
- Ostrom QT, Gittleman H, de Blank PM, Finlay JL, Gurney JG, McKean-Cowdin R, Stearns DS, Wolff JE, Liu M, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. American Brain Tumor Association Adolescent and Young Adult Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008-2012. Neuro Oncol. 2016 Jan;18 Suppl 1(Suppl 1):i1-i50. doi: 10.1093/neuonc/nov297. PMID: 26705298; PMCID: PMC4690545.
- Dietrich J, Monje M, Wefel J, Meyers C. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist. 2008 Dec;13(12):1285-95. doi: 10.1634/theoncologist.2008-0130. Epub 2008 Nov 19. PMID: 19019972.
- Jaggi AS, Singh N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology. 2012 Jan 27;291(1-3):1-9. doi: 10.1016/j.tox.2011.10.019. Epub 2011 Nov 3. PMID: 22079234.
- Rivera E, Cianfrocca M. Overview of neuropathy associated with taxanes for the treatment of metastatic breast cancer. Cancer Chemother Pharmacol. 2015 Apr;75(4):659-70. doi: 10.1007/s00280-014-2607-5. Epub 2015 Jan 18. PMID: 25596818; PMCID: PMC4365177.
- Hansen N. Drug-induced encephalopathy. In: Tanasescu R, editor. Miscellanea on encephalopathies a second look. InTech. 2012; 39-60.
- DeAngelis LM, Posner JB. Side effects of chemotherapy. In: Neurologic complications of cancer, 2nd. New York, Oxford University Press. 2009; 447.
- Bhojwani D, Sabin ND, Pei D, Yang JJ, Khan RB, Panetta JC, Krull KR, Inaba H, Rubnitz JE, Metzger ML, Howard SC, Ribeiro RC, Cheng C, Reddick WE, Jeha S, Sandlund JT, Evans WE, Pui CH, Relling MV. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol. 2014 Mar 20;32(9):949-59. doi: 10.1200/JCO.2013.53.0808. Epub 2014 Feb 18. PMID: 24550419; PMCID: PMC3948096.
- Li SH, Chen WH, Tang Y, Rau KM, Chen YY, Huang TL, Liu JS, Huang CH. Incidence of ischemic stroke post-chemotherapy: a retrospective review of 10,963 patients. Clin Neurol Neurosurg. 2006 Feb;108(2):150-6. doi: 10.1016/j.clineuro.2005.03.008. Epub 2005 Apr 25. PMID: 16412836.
- Swain SM, Arezzo JC. Neuropathy associated with microtubule inhibitors: diagnosis, incidence, and management. Clin Adv Hematol Oncol. 2008 Jun;6(6):455-67. PMID: 18567992.