More Information
Submitted: March 30, 2023 | Approved: April 07, 2023 | Published: April 10, 2023
How to cite this article: Khan R, Selehria A, Aquil H, Sheraz A, Khan S, et al. Diagnostic accuracy of apparent diffusion coefficient (ADC) in differentiating low- and high-grade gliomas, taking histopathology as the gold standard. J Radiol Oncol. 2023; 7: 013-019.
DOI: 10.29328/journal.jro.1001047
Copyright License: © 2023 Khan R, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Diagnostic accuracy of apparent diffusion coefficient (ADC) in differentiating low- and high-grade gliomas, taking histopathology as the gold standard
Raheel Khan, Atiq-ur-Rehman Selehria, Hafsa Aquil, Atif Sheraz, Sara Khan, Najwa Zahoor and Anashia Kayani*
Armed Forces institute of Radiology and Imaging, Islamabad, Pakistan
*Address for Correspondence: Anashia Kayani, Armed Forces institute of Radiology and Imaging, Islamabad, Pakistan, Email: [email protected]
Gliomas are known to be one of the most grievous malignant central nervous system (CNS) tumors and have a high mortality rate with a low survival rate severe disability and increase risk of recurrence. Aim of his study is to determine the diagnostic accuracy of apparent diffusion coefficient (ADC) in differentiating low-grade and high-grade gliomas, taking histopathology as the gold standard. It is a Cross-sectional validation study conducted at the Armed Forces Institute of Radiology and Imaging, (AFIRI) Rawalpindi, Pakistan from 28th February 2022 to 27th August 2022.
Materials and methods: A total of 215 patients with focal brain lesions of age 25-65 years of either gender were included. Patients with a cardiac pacemaker, breastfeeding females, de-myelinating lesions and malignant infiltrates, and renal failure were excluded. Then diffusion-weighted magnetic resonance imaging was performed on each patient by using a 1.5 Tesla MR system. The area of greatest diffusion restriction (lowest ADC) within the solid tumor component was identified while avoiding areas of peritumoral edema. Results of ADC were interpreted by a consultant radiologist (at least 5 years of post-fellowship experience) for high or low-grade glioma. After this, each patient has undergone a biopsy in the concerned ward, and histopathology results were compared with ADC findings.
Results: Overall sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of apparent diffusion coefficient (ADC) in differentiating low- and high-grade gliomas, taking histopathology as the gold standard was 93.65%, 87.64%, 91.47%, 90.70% and 91.16% respectively.
Conclusion: This study concluded that apparent diffusion coefficient (ADC) is the non-invasive modality of choice with high diagnostic accuracy in differentiating low- and high-grade gliomas.
Gliomas are known to be one of the most grievous malignant central nervous system (CNS) tumors and have a high mortality rate with a low survival rate severe disability and increase risk of recurrence [1]. An annual incidence rate of glioma in the United States is about 5 in 100,000 population and represents 4.9% of all types of cancer cases [2]. Glioma refers to tumors that have a histological feature of glial cells (including oligodendrocytes, astrocytes, and ependymal cells). According to the WHO classification, they are divided into four grades based on neoangiogenesis, nuclear pleomorphism, degree of mass effect and perifocal edema and cellularity. Table AA shows the grades of glioma based on histological subtypes.
| Table AA: The grades of glioma based on histological subtypes | |||
| WHO grade | Histology | ||
| Grade I | Pilocytic astrocytoma | ||
| Grade II | Diffuse astrocytoma | Oligoastrocytoma | Oligodendroglioma |
| Grade III | Anaplastic astrocytoma | Anaplastic Oligoastrocytoma | Anasplastic Oligodendroglioma |
| Grade IV | Glioblastoma Multiformi | ||
The most aggressive grade is glioblastoma multiform (grade IV), which accounts for 47% of malignant CNS tumors, and its prognosis is the worst among all cancers with five years survival rate of merely 5.5% [3].
The exact pathogenesis of gliomas is idiopathic. Hereditary genetic disorders such as neurofibromatoses (type 1 and type 2) and tuberous sclerosis complex are known to predispose to their development [4,5]. Different oncogenes can cooperate in the development of gliomas [6]. Gliomas have been correlated to the electromagnetic radiation from cell phones, and a link between cancer and cell phone usage was considered possible, though several large studies have found no conclusive evidence. Experiments designed to test such a link gave negative results [7]. Most glioblastomas are infected with cytomegalovirus, which speeds the development of tumors [8-10]. Over the past few years, MRI has been evaluated as the gold standard for imaging CNS. It has immensely improved the detection rate due to its high resolution and precision in determining the margin of tumor and therefore enhancing the resection accuracy resulting in an improvement in the survival rate [4]. Regardless of the ongoing advances in modern imaging techniques and medical management the survival and prognosis of a patient diagnosed with glioma are very grim with poor prognosis [5].
Diffusion magnetic resonance imaging (dMRI) of the human brain, first described in 1985, has become an integral part of neuroimaging [6]. Apparent diffusion coefficient (ADC) is a modality based on the diffusion properties of water molecules within tissues at anatomically three levels. The pattern of diffusion of water molecules is quite different from the Brownian motion pattern in brain parenchyma as the water molecules have to cross the blood-brain barrier and interact with tissue components such as cell membranes, cell fibers, and macromolecules [7]. Due to the heterogeneity of brain microarchitecture, normal and diseased areas of the brain have different ADCs. Tissues with higher rates of water molecules diffusivity, reduced cellularity, and compact mass have higher ADCs [8]. ADC, therefore, characterizes tissues quantitatively, and findings are validated in several studies [9,10]. A study has shown the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of diffusion-weighted imaging in differentiating high and low-grade gliomas as 89%, 100%, 100%, 86%, and 93% respectively [9]. Another study has shown the prevalence of high-grade gliomas as 31.58% and sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) of ADC values in differentiation between low- and high-grade gliomas as 77.6%, 80.3%, 78.5%, 89.5% and 62.4% respectively [10].
Magnetic resonance imaging (MRI) is considered to be the imaging modality of choice to first assess CNS tumors such as gliomas. With the advancement of technology, many more physiological MRI techniques including MR spectroscopy, diffusion-weighted imaging (DWI), and perfusion-weighted imaging (PWI) are currently being used as important tools for staging and classification of gliomas [11]. The Apparent diffusion Coefficient (ADC) is an imaging modality with is a derivative of DWI that is pessimistically correlated with cell proliferation indices and shown to have increasing potential as a noninvasive imaging biomarker for preoperative tumor grading [12,13]. Various studies performed have scrutinized the role of DWI with quantitative ADC in the differentiation between high-grade glioma from low-grade glioma [14,15].
The rationale of this study was to determine the diagnostic accuracy of apparent diffusion coefficient (ADC) in differentiating low- and high-grade gliomas, taking histopathology as the gold standard. If ADC diagnostic accuracy will be found high in differentiating low- and high-grade gliomas then this non-invasive modality can be applied routinely in our general practice for proper treatment selection to reduce the morbidity and mortality of these particular patients.
The study was conducted at the Armed Forces Institute of Radiology and Imaging, Rawalpindi from 28th February 2022 to 27th August 2022. After approval from the institutional ethical review committee (IERB approval certificate no.0058). The sample size was calculated by using open epi calculator (https://www.openepi.com/SampleSize/SSPropor.htm) and having a 95% confidence level with an expected prevalence of high-grade glioma as 31.58% with 10% absolute precision for sensitivity and specificity of ADC value in differentiating high grade and low-grade glioma as 77.6% and 80.3% respectively [8]. 215 patients with a mean age of 41.27 ± 9.45 years (25-65 years) presenting to the Armed Forces Institute of Radiology And Imaging, Rawalpindi, fulfilling the inclusion criteria were selected. Informed consent was taken from each patient. Then diffusion-weighted magnetic resonance imaging was performed on each patient by using a 1.5 Tesla MR system. The area of greatest diffusion restriction having a high DWI value and lowest ADC value within the solid tumor component was identified and surrounding peri-tumoral edema was not included. Areas of obviously restricted diffusion within each tumor which are defined as areas of bright signal intensity on b1000 images and appearing corresponding to a dark area on the ADC map were recognized. The first step in numeric ADC analysis was to study the T1, T2 and post-gadolinium sequences first to visualize and avoid areas likely to be cystic, hemorrhagic, calcific, necrotic, or peritumoral edema. The slice containing the lowest value of ADC was identified as a region of interest (ROI) and was selected with the help of the eclipse tool with an area of 0.16 cm2 and using MRI in build software ADC value was calculated and recorded. Results of ADC were interpreted by a consultant radiologist (at least 5 years of post-fellowship experience) for high or low-grade glioma (less than 800 × 10^-6mm2/sec as high grade and more than 800 × 10^-6mm2/sec as low-grade glioma). After this, each patient has undergone a biopsy of the brain tumor which was done mainly in the center of tumor mass/ predominantly solid component in the concerned ward, and histopathology results were compared with ADC findings. This all data was recorded on a specially designed proforma.
Inclusion criteria
1. All patients with focal brain lesions with radiological features of glioma
2. Size of lesion >5mm.
3. Age 25-65 years.
4. Both genders.
Exclusion criteria
1. Pregnant or lactating females as assessed on history.
2. Patients with a cardiac pacemaker.
3. Patients with renal failure (assessed on history and s/creatinine >1.5 mg/dl).
4. Purely cystic lesions, including abscesses.
5. Demyelinating lesions and malignant infiltrates.
6. Choroid plexus tumor, mesenchymal and non-meningothelial tumors of the sellar region, and pineal region tumor.
7. Patients with a known history of primary malignancy with suspicion of metastasis to the brain.
Collected data was analyzed through computer software SPSS 25.0. Mean and standard deviation was noted for the age and size of the lesion. Frequency and percentage were calculated for gender, site of lesion, and low or high-grade gliomas on ADC and histopathology. A 2×2 contingency table was used to calculate the sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of apparent diffusion coefficient (ADC) in differentiating low- and high-grade gliomas, taking histopathology as the gold standard. Stratification was done for age, gender, site of lesion, and size of the lesion, and post-stratification diagnostic accuracy of apparent diffusion coefficient (ADC) was calculated.
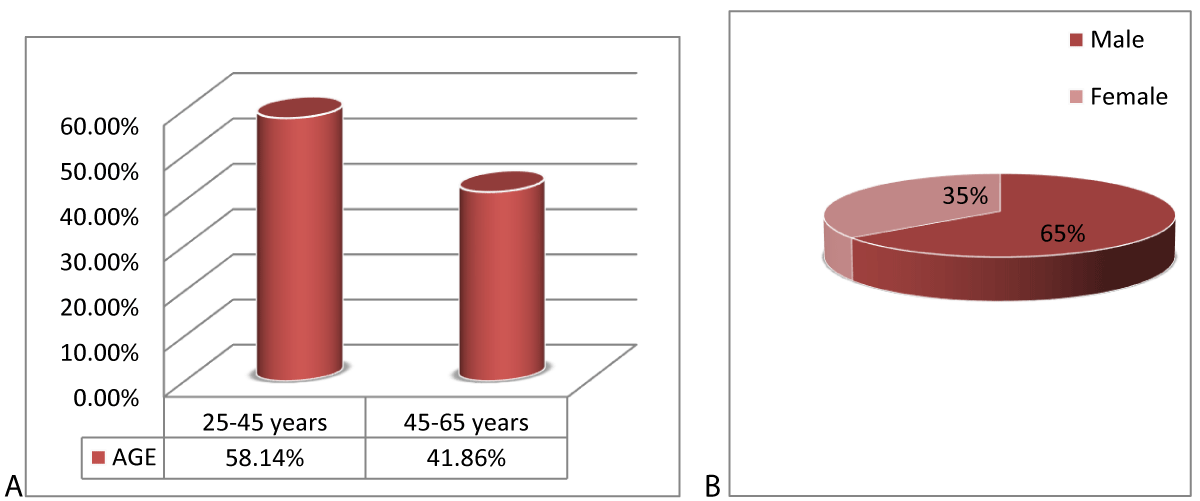
The age range in this study was from 25-65 years with a mean age of 41.27 ± 9.45 years. The majority of the patients 125 (58.14%) were between 25 to 45 years of age as shown in Figure 1. Out of these 215 patients, 139 (64.65%) were male and 76 (35.35%) were females with a ratio of 1.8:1 (Figure 1). The mean size of the lesion was 2.12 ± 1.17 cm. Distribution of patients according to the site of the lesion is shown in Table 1.
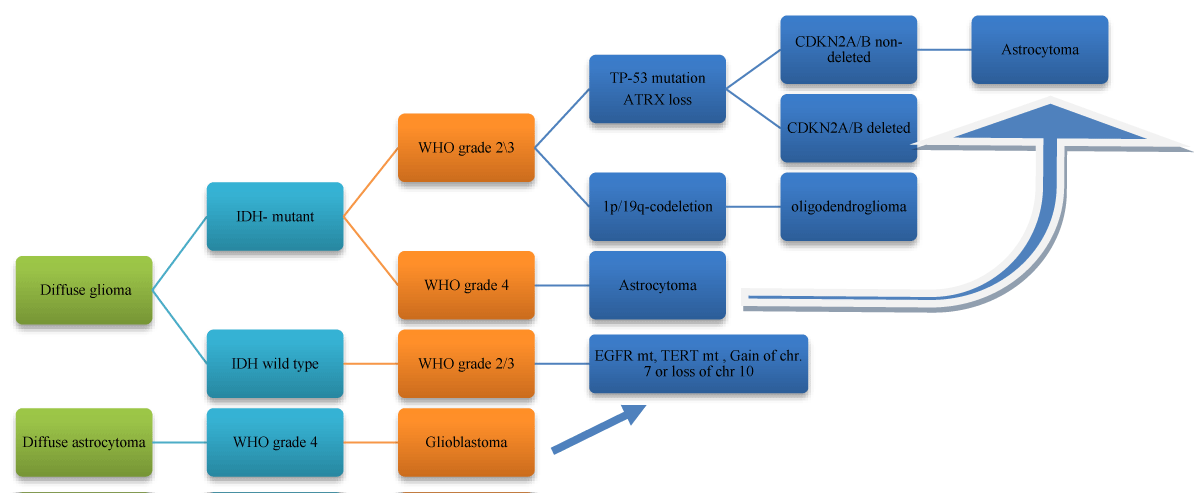
Figure 1: WHO classification of grades of glioma according to histology and molecular features (a) simple histological classification (b) Algorithm based on 2021 WHO classification of brain tumors.
| Table 1: Distribution of patients according to the site of the lesion. | ||
| Site of the lesion (cm) | No. of Patients | %age |
| Supra-tentorial | 156 | 72.56 |
| Infra-tentorial | 59 | 27.44 |
| Total | 215 | 100.0 |
All the patients were subjected to the apparent diffusion coefficient (ADC) of the brain. In ADC-positive patients, 118 were True Positive and 11 were False Positive. Among 86, ADC-negative patients, 08 were False Negative whereas 78 were True Negative as shown in Table 2. Overall sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of apparent diffusion coefficient (ADC) in differentiating low-and high-grade gliomas, taking histopathology as the gold standard was 93.65%, 87.64%, 91.47%, 90.70%, and 91.16% respectively.
| Table 2: Diagnostic accuracy of apparent diffusion coefficient (ADC) in differentiating low- and high-grade gliomas, taking histopathology as the gold standard. | |||
| The high grade in Histopathology | Low grade on Histopathology | p - value | |
| High Grade on ADC | 118 (TP)* | 11 (FP)*** | 0.0001 |
| Low grade on ADC | 08 (FN)** | 78 (TN)**** | |
| *-TP: True Positive; **-FN: False negative ***; FP: False Positive; ****-TN: True Negative Sensitivity: 93.65%, Specificity: 87.64%, PPV : Positive Predictive Value 91.47%; NPV: Negative Predictive Value 90.70% and Diagnostic Accuracy: 91.16% | |||
Stratification of diagnostic accuracy concerning the size of lesion < 3 cm (n = 176) showed that 97 were True Positive and 09 were False Positive, among the remaining 70 patients low-grade patients on ADC, 08 (False Negative) had high-grade glioma on histopathology whereas 62 (True Negative) had no low-grade glioma on histopathology with the sensitivity of 92.38%, specificity 87.32%, positive predictive value (PPV) of 91.51%, negative predictive value (NPV) of 88.57% and diagnostic accuracy of 90.34%. Stratification of diagnostic accuracy concerning the size of lesion greater than 3 cm (n = 39) showed that 21 were True Positive and 02 were False Positive, among the remaining 16 patients low-grade patients on ADC, 0 (False Negative whereas 16 (True Negative) had no low-grade glioma on histopathology with the sensitivity of 100%, specificity 88.89%, positive predictive value (PPV) of 91.30%, negative predictive value (NPV) of 100% and diagnostic accuracy of 94.87%. Stratification of diagnostic accuracy concerning supra-tentorial (n = 156) showed that 94 were True Positive and 04 were False Positive, among the remaining 58 patients low-grade patients on ADC, 02 (False Negative) had high-grade glioma on histopathology whereas 56 (True Negative) had no low-grade glioma on histopathology with a sensitivity of 97.92%, specificity 93.33%, positive predictive value (PPV) of 95.92%, negative predictive value (NPV) of 96.55% and diagnostic accuracy of 96.15%. Stratification of Diagnostic accuracy concerning infra-tentorial (n = 59) showed that 24 were True Positive and 07 were False Positive, among the remaining 58 patients low-grade patients on ADC, 06 (False Negative) had high-grade glioma on histopathology whereas 22 (True Negative) had no low-grade glioma on histopathology with the sensitivity of 80.0%, specificity 75.86%, positive predictive value (PPV) of 77.42%, negative predictive value (NPV) of 78.57% and diagnostic accuracy of 77.97% Tables 3,4, Figure 2.
| Table 3: Stratification of diagnostic accuracy concerning age 25-45 years (n = 125). | |||
| The high grade in Histopathology | Low grade on Histopathology | p - value | |
| High Grade on ADC | 67 (TP) | 04 (FP) | 0.001 |
| Low grade on ADC | 03 (FN) | 51 (TN) | |
| Sensitivity: 95.71% ; Specificity: 92.73%; PPV : Positive Predictive Value 94.37%; NPV: Negative Predictive Value 94.44%; Diagnostic Accuracy: 94.40% | |||
| Table 4: Stratification of diagnostic accuracy concerning age 46-65 years (n = 90). | |||
| The high grade in Histopathology | Low grade on Histopathology | p - value | |
| High Grade on ADC | 51 (TP) | 07 (FP) | 0.001 |
| Low grade on ADC | 05 (FN) | 27 (TN) | |
| Sensitivity: 91.07%; Specificity: 79.41%; PPV: Positive Predictive Value 87.93%; NPV: Negative Predictive Value 84.38%; Diagnostic Accuracy: 86.67% | |||
Figure 2: Distribution of patients according to (A) age and (B) Gender (n = 215).
We have conducted this study to determine the diagnostic accuracy of Apparent Diffusion Coefficient (ADC) in differentiating low- and high-grade gliomas, taking histopathology as the gold standard. Overall sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of apparent diffusion coefficient (ADC) in differentiating low- and high-grade gliomas, taking histopathology of a brain tumor as the gold standard was 93.65%, 87.64%, 91.47%, 90.70% and 91.16% respectively Figures 3,4.
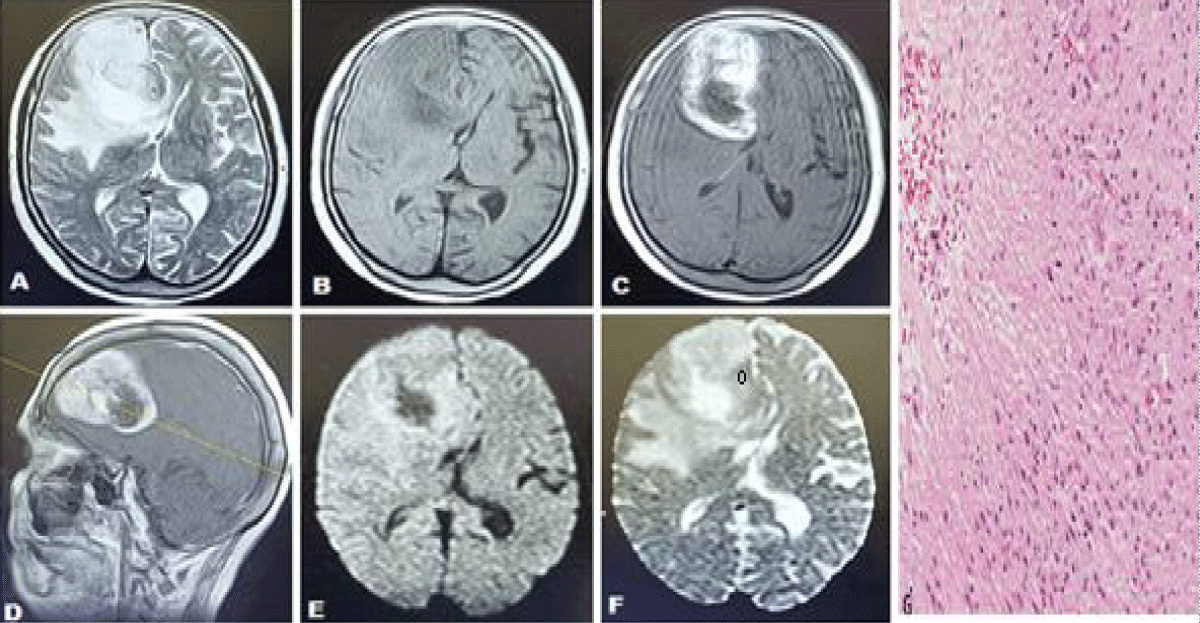
Figure 3: Case of 56 years old ale presenting with headache and left-sided body weakness (high-grade glioma). Axial T2 weighted sequences (T2WS) (A) axial T1 weighted sequences (T1WS) (B) axial and sagittal post-contrast T1WS (C, D) shows an abnormal signal intensity lesion in the high front temporal lobe extending into genu of the corpus callosum and basal ganglia appearing hypointense on T1WS and hyperintense on T2W sequences with mass effect and edema, demonstrating enhancement on post contrast images (C, D) along with a restriction on Diffusion-weighted images (DWI) (E) and ADC map (F) with a low ADC value of 137 x 10-6 mm2 /sec. (Favoring high-grade glioma). (G) Histopathology showed necrosis and sheets of irregular or elongated cells with pleomorphic nuclei consistent with glioblastoma area.
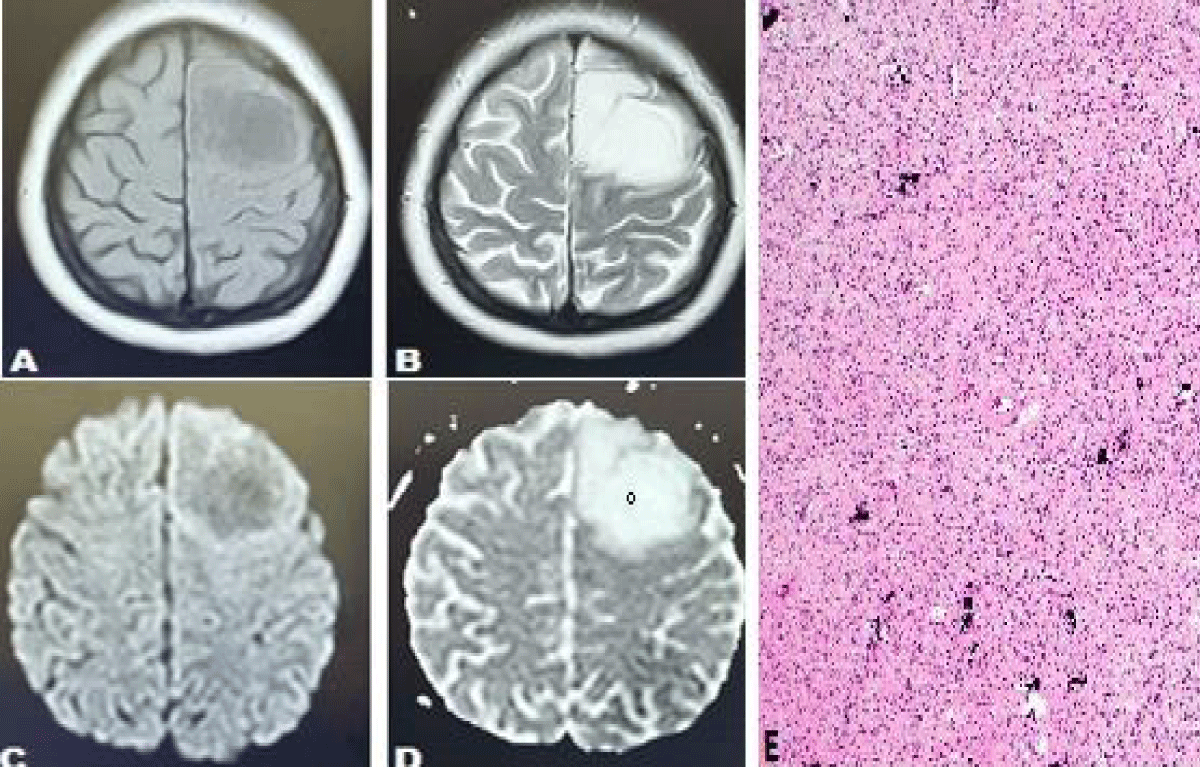
Figure 4: Case of 44 years female presented with headache and an episode of seizures. Axial T1 weighted sequences (T1WS) (A) and T2 weighted sequences (T2WS) (B) show an abnormal signal intensity lesion in the left frontal lobe appearing hypointense on T1WS and hyperintense on T2W sequences and demonstrating facilitated diffusion on Diffusion-weighted images (DWI) (C) and ADC map (D) with a high ADC value of 1123 x 10-6 mm2 /sec. (demonstrating low-grade glioma). (E) Histopathology report showed scattered microcalcifications which are characteristic of oligodendroglioma.
A study conducted by Rong Y in 2021 has shown sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of diffusion-weighted imaging in differentiating high and low-grade gliomas as 89%, 100%, 100%, 86% and 93% respectively [9]. Another study conducted by Abd EL-salam SM in 2019 has shown the prevalence of high grade gliomas31.58% and sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) of ADC values in differentiation between low- and high-grade gliomas as 77.6%, 80.3%, 78.5%, 89.5% and 62.4% respectively [10].
In a meta-analysis which is published based on two kinds of MRI techniques such as diffusion-weighted imaging and diffusion tensor imaging for glioma classification, the total pooled overall accuracy (AUC) of 0.90, with a sensitivity of 85% and 80% [16]. In another meta-analysis which was performed on perfusion-weighted images for grading of glioma showed to have a sensitivity of 93%, and specificity of 81% with a diagnostic odd ratio of 55% [17]. The results demonstrated that perfusion-weighted images (PWI) are also a useful tool for differentiating glioma, however, this is performed by injecting contrast medium with many other influencing factors which makes it difficult to generalize the method. In view of evidence found from a comprehensive meta-analysis, the diagnostic accuracy of DWI in grading glioma is considered to be less than MR spectroscopy, DSC and DCE MRI [18]. Advantage of performing diffusion-weighted imaging over all other techniques of MR is being very easily accessible, no need of any contrast material, non-radioactive as well as comparatively cost-effective therefore is easy to ubiquitous exercise. Additionally, with the DWI and ADC techniques combined with other MR imaging such as PWI/DCE/MRS, the accuracy will be significantly improved [19].
Arvinda, et al. [20] have studied 51 patients (age range, 28–58 years; median age, 40 years) who were diagnosed with gliomas and undergo perfusion and diffusion MR imaging. They discovered that relative cerebral blood volume along with ADC and ADC ratio individually or in combination were helpful tools in preoperative glioma grading.
In addition, Kang, et al. [21] declared that histogram analysis based on ADC maps of the entire tumor volume is a useful tool for assessing glioma grade. However, other studies, such as Lam, et al. [22] concluded that there was no significant difference in ADC values between low- and high-grade gliomas. This discrepancy may be related to heterogeneous tumor structures in the glioma and the different methods used for measuring ADC values. The AGEs may be used to analyze ADCs of the enhancing part of the tumor, the entire volume of the tumor, or the darkest region of the tumor on the ADC map in the reports, each of which provides different information about the heterogeneity and tissue characteristics of the tumors. Provenzale, et al. [23] considered whether the analyses of tumor ADC excluded areas of necrosis, which may have resulted in the conflicting findings.
Koral, et al. [24], studied 140 patients (58 medullo-blastomas, 10 AT/RT, 51 astrocytomas, 21 ependymomas) and could differentiate astrocytoma from ependymoma with 78% sensitivity and 78% specificity using an ADC ratio ≥ 1.8 and could differentiate embryonal tumors from ependymoma with 87% sensitivity and 83% specificity using an ADC ratio ≤ 1.2. Also, Zitouni, et al. [25] differentiated astrocytomas from ependymoma with 85.7% sensitivity and 90% specificity using an ADC ratio ≥ 1.7 and differentiated medulloblastoma from ependymoma with 100% sensitivity and 88.89% specificity using an ADC ratio ≤ 1.18, which agreed with our results. Gimi et al [26] studied 79 patients (31 JPA, 27 medulloblastomas, 14 ependymoma, 7 AT/RT) and measured the ADC ratios as 2.30, 1.58, 0.97, and 0.83 for astrocytoma, ependymoma, medulloblastoma, and AT/RT. They differentiated astrocytoma from ependymoma with 92% sensitivity and 79% specificity using an ADC ratio ≥ 1.7 and differentiated embryonal tumors (medulloblastoma and AT/RT) from ependymoma with 93% sensitivity and 88% specificity using an ADC ratio ≤ 1.2.
Fan, et al. [27] evaluated the utility of DWI in patients with non-enhancing supratentorial brain gliomas. They also found that ADC values calculated from the tumor core were helpful in differentiating and grading non-enhancing gliomas, but their subjects included patients having tumors with heterogeneous signal intensity and clear evidence of central necrosis on conventional MR imaging. Therefore, their subjects differed from those in our study. Studies by Rollin, et al. [28] and Lam, et al. [29] failed to find a significant difference between the ADC values of high-grade and low-grade gliomas, and some studies have shown that tumor minimum ADC values have preoperative prognostic importance in patients with malignant supratentorial astrocytomas [30,31]. In addition, Barker, et al. [32] and Scott, et al. [33] demonstrated that the risk of anaplasia in non-enhancing cerebral tumors increases with age, whereas we did not find any difference between the ages of the patients with high- and lowgrade astrocytomas.
This study concluded that apparent diffusion coefficient (ADC) is the non-invasive modality of choice with high diagnostic accuracy in differentiating low- and high-grade gliomas, and has not only dramatically improved our ability to differentiate low- and high-grade gliomas pre-operatively but also helps the surgeons for proper decision making. So, we recommend that apparent diffusion coefficient (ADC) should be done routinely in all cases of cerebral gliomas for accurate assessment pre-operatively and opting proper surgical approach and reducing pure diagnostic biopsies in cerebral gliomas which ultimately reduce the morbidity and mortality of these patients.
- Al-Agha M, Abushab K, Quffa K, Al-Agha S, Alajerami Y, Tabash M. Efficiency of High and Standard b Value Diffusion-Weighted Magnetic Resonance Imaging in Grading of Gliomas. J Oncol. 2020 Sep 14;2020:6942406. doi: 10.1155/2020/6942406. PMID: 33005190; PMCID: PMC7509551.
- Wesseling P, Capper D. WHO 2016 Classification of gliomas. Neuropathol Appl Neurobiol. 2018 Feb;44(2):139-150. doi: 10.1111/nan.12432. PMID: 28815663.
- Fawzy FM, Almassry HN, Ismail YM. Preoperative glioma grading by MR diffusion and MR spectroscopic imaging. Egyptian Journal of Radiology and Nuclear Medicine (Online). 2016; 47(4): 1539-1548.
- Zhang L, Min Z, Tang M, Chen S, Lei X, Zhang X. The utility of diffusion MRI with quantitative ADC measurements for differentiating high-grade from low-grade cerebral gliomas: Evidence from a meta-analysis. J Neurol Sci. 2017 Feb 15;373:9-15. doi: 10.1016/j.jns.2016.12.008. Epub 2016 Dec 9. Erratum in: J Neurol Sci. 2017 Apr 15;375:103-106. PMID: 28131237.
- Darbar A, Waqas M, Enam SF, Mahmood SD. Use of Preoperative Apparent Diffusion Coefficients to Predict Brain Tumor Grade. Cureus. 2018 Mar 7;10(3):e2284. doi: 10.7759/cureus.2284. PMID: 29740523; PMCID: PMC5938001.
- Qin JB, Zhang H, Wang XC, Tan Y, Wu XF. Combination value of diffusion-weighted imaging and dynamic susceptibility contrast-enhanced MRI in astrocytoma grading and correlation with GFAP, Topoisomerase IIα and MGMT. Oncol Lett. 2019 Sep;18(3):2763-2770. doi: 10.3892/ol.2019.10656. Epub 2019 Jul 24. PMID: 31452754; PMCID: PMC6704283.
- Zhang L, Min Z, Tang M, Chen S, Lei X, Zhang X. The utility of diffusion MRI with quantitative ADC measurements for differentiating high-grade from low-grade cerebral gliomas: Evidence from a meta-analysis. J Neurol Sci. 2017 Feb 15;373:9-15. doi: 10.1016/j.jns.2016.12.008. Epub 2016 Dec 9. Erratum in: J Neurol Sci. 2017 Apr 15;375:103-106. PMID: 28131237.
- Al-Sharydah AM, Al-Arfaj HK, Saleh Al-Muhaish H, Al-Suhaibani SS, Al-Aftan MS, Almedallah DK, Al-Abdulwahhab AH, Al-Hedaithy AA, Al-Jubran SA. Can apparent diffusion coefficient values help distinguish between different types of pediatric brain tumors? Eur J Radiol Open. 2019 Jan 4;6:49-55. doi: 10.1016/j.ejro.2018.12.004. PMID: 30627595; PMCID: PMC6321863.
- Yao R, Cheng A, Liu M, Zhang Z, Jin B, Yu H. The Diagnostic Value of Apparent Diffusion Coefficient and Proton Magnetic Resonance Spectroscopy in the Grading of Pediatric Gliomas. J Comput Assist Tomogr. 2021 Mar-Apr 01;45(2):269-276. doi: 10.1097/RCT.0000000000001130. PMID: 33346568; PMCID: PMC7972297.
- Abd El-Salam SM, Mokhtar O. Diagnostic accuracy of diffusion-weighted imaging in evaluation of high and low-grade pediatric brain tumors. Medical Journal of Cairo University. 2019; 87(3):1877-83. DOI:10.21608/mjcu.2019.54033
- Ryu YJ, Choi SH, Park SJ, Yun TJ, Kim JH, Sohn CH. Glioma: application of whole-tumor texture analysis of diffusion-weighted imaging for the evaluation of tumor heterogeneity. PLoS One. 2014 Sep 30;9(9):e108335. doi: 10.1371/journal.pone.0108335. PMID: 25268588; PMCID: PMC4182447.
- Guo AC, Cummings TJ, Dash RC, Provenzale JM. Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology. 2002 Jul;224(1):177-83. doi: 10.1148/radiol.2241010637. PMID: 12091680.
- Higano S, Yun X, Kumabe T, Watanabe M, Mugikura S, Umetsu A, Sato A, Yamada T, Takahashi S. Malignant astrocytic tumors: clinical importance of apparent diffusion coefficient in prediction of grade and prognosis. Radiology. 2006 Dec;241(3):839-46. doi: 10.1148/radiol.2413051276. Epub 2006 Oct 10. PMID: 17032910.
- Chen Z, Ma L, Lou X, Zhou Z. Diagnostic value of minimum apparent diffusion coefficient values in prediction of neuroepithelial tumor grading. J Magn Reson Imaging. 2010 Jun;31(6):1331-8. doi: 10.1002/jmri.22175. PMID: 20512884.
- Wu CC, Guo WY, Chen MH, Ho DM, Hung AS, Chung HW. Direct measurement of the signal intensity of diffusion-weighted magnetic resonance imaging for preoperative grading and treatment guidance for brain gliomas. J Chin Med Assoc. 2012 Nov;75(11):581-8. doi: 10.1016/j.jcma.2012.08.019. Epub 2012 Nov 2. PMID: 23158036.
- Zhang L, Min Z, Tang M, Chen S, Lei X, Zhang X. The utility of diffusion MRI with quantitative ADC measurements for differentiating high-grade from low-grade cerebral gliomas: Evidence from a meta-analysis. J Neurol Sci. 2017 Feb 15;373:9-15. doi: 10.1016/j.jns.2016.12.008. Epub 2016 Dec 9. Erratum in: J Neurol Sci. 2017 Apr 15;375:103-106. PMID: 28131237.
- Min ZG, Liu HJ, Li M, Liu LH, Jin CW, Zhang M. [Accuracy of MR perfusion weighted imaging for cerebral glioma grading: a meta-analysis]. Zhonghua Yi Xue Za Zhi. 2010 Nov 9;90(41):2927-31. Chinese. PMID: 21211399.
- van Dijken BRJ, van Laar PJ, Holtman GA, van der Hoorn A. Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with high-grade glioma, a systematic review and meta-analysis. Eur Radiol. 2017 Oct;27(10):4129-4144. doi: 10.1007/s00330-017-4789-9. Epub 2017 Mar 22. PMID: 28332014; PMCID: PMC5579204.
- Wang QP, Lei DQ, Yuan Y, Xiong NX. Accuracy of ADC derived from DWI for differentiating high-grade from low-grade gliomas: Systematic review and meta-analysis. Medicine (Baltimore). 2020 Feb;99(8):e19254. doi: 10.1097/MD.0000000000019254. PMID: 32080132; PMCID: PMC7034741.
- Arvinda HR, Kesavadas C, Sarma PS, Thomas B, Radhakrishnan VV, Gupta AK, Kapilamoorthy TR, Nair S. Glioma grading: sensitivity, specificity, positive and negative predictive values of diffusion and perfusion imaging. J Neurooncol. 2009 Aug;94(1):87-96. doi: 10.1007/s11060-009-9807-6. Epub 2009 Feb 20. Retraction in: J Neurooncol. 2013 Sep;114(2):255. PMID: 19229590.
- Kang Y, Choi SH, Kim YJ, Kim KG, Sohn CH, Kim JH, Yun TJ, Chang KH. Gliomas: Histogram analysis of apparent diffusion coefficient maps with standard- or high-b-value diffusion-weighted MR imaging--correlation with tumor grade. Radiology. 2011 Dec;261(3):882-90. doi: 10.1148/radiol.11110686. Epub 2011 Oct 3. PMID: 21969667.
- Lam WW, Poon WS, Metreweli C. Diffusion MR imaging in glioma: does it have any role in the pre-operation determination of grading of glioma? Clin Radiol. 2002 Mar;57(3):219-25. doi: 10.1053/crad.2001.0741. PMID: 11952318.
- Provenzale JM, Mukundan S, Barboriak DP. Diffusion-weighted and perfusion MR imaging for brain tumor characterization and assessment of treatment response. Radiology. 2006 Jun;239(3):632-49. doi: 10.1148/radiol.2393042031. PMID: 16714455.
- Koral K, Alford R, Choudhury N, Mossa-Basha M, Gargan L, Gimi B, Gao A, Zhang S, Bowers DC, Koral KM, Izbudak I. Applicability of apparent diffusion coefficient ratios in preoperative diagnosis of common pediatric cerebellar tumors across two institutions. Neuroradiology. 2014 Sep;56(9):781-8. doi: 10.1007/s00234-014-1398-z. Epub 2014 Jun 29. PMID: 24974083.
- Zitouni S, Koc G, Doganay S, Saracoglu S, Gumus KZ, Ciraci S, Coskun A, Unal E, Per H, Kurtsoy A, Kontas O. Apparent diffusion coefficient in differentiation of pediatric posterior fossa tumors. Jpn J Radiol. 2017 Aug;35(8):448-453. doi: 10.1007/s11604-017-0652-9. Epub 2017 May 26. PMID: 28550357.
- Gimi B, Cederberg K, Derinkuyu B, Gargan L, Koral KM, Bowers DC, Koral K. Utility of apparent diffusion coefficient ratios in distinguishing common pediatric cerebellar tumors. Acad Radiol. 2012 Jul;19(7):794-800. doi: 10.1016/j.acra.2012.03.004. Epub 2012 Apr 17. PMID: 22513110.
- Fan GG, Deng QL, Wu ZH, Guo QY. Usefulness of diffusion/perfusion-weighted MRI in patients with non-enhancing supratentorial brain gliomas: a valuable tool to predict tumour grading? Br J Radiol. 2006 Aug;79(944):652-8. doi: 10.1259/bjr/25349497. Epub 2006 Apr 26. PMID: 16641420.
- Rollin N, Guyotat J, Streichenberger N, Honnorat J, Tran Minh VA, Cotton F. Clinical relevance of diffusion and perfusion magnetic resonance imaging in assessing intra-axial brain tumors. Neuroradiology. 2006 Mar;48(3):150-9. doi: 10.1007/s00234-005-0030-7. Epub 2006 Feb 10. PMID: 16470375.
- Lam WW, Poon WS, Metreweli C. Diffusion MR imaging in glioma: does it have any role in the pre-operation determination of grading of glioma? Clin Radiol. 2002 Mar;57(3):219-25. doi: 10.1053/crad.2001.0741. PMID: 11952318.
- Murakami R, Sugahara T, Nakamura H, Hirai T, Kitajima M, Hayashida Y, Baba Y, Oya N, Kuratsu J, Yamashita Y. Malignant supratentorial astrocytoma treated with postoperative radiation therapy: prognostic value of pretreatment quantitative diffusion-weighted MR imaging. Radiology. 2007 May;243(2):493-9. doi: 10.1148/radiol.2432060450. Epub 2007 Mar 13. PMID: 17356177.
- Oh J, Henry RG, Pirzkall A, Lu Y, Li X, Catalaa I, Chang S, Dillon WP, Nelson SJ. Survival analysis in patients with glioblastoma multiforme: predictive value of choline-to-N-acetylaspartate index, apparent diffusion coefficient, and relative cerebral blood volume. J Magn Reson Imaging. 2004 May;19(5):546-54. doi: 10.1002/jmri.20039. PMID: 15112303.
- Barker FG 2nd, Chang SM, Huhn SL, Davis RL, Gutin PH, McDermott MW, Wilson CB, Prados MD. Age and the risk of anaplasia in magnetic resonance-nonenhancing supratentorial cerebral tumors. Cancer. 1997 Sep 1;80(5):936-41. PMID: 9307194.
- Scott JN, Brasher PM, Sevick RJ, Rewcastle NB, Forsyth PA. How often are nonenhancing supratentorial gliomas malignant? A population study. Neurology. 2002 Sep 24;59(6):947-9. doi: 10.1212/wnl.59.6.947. PMID: 12297589.